Acute myeloid leukemia
This article needs to be updated. (July 2021) |
| Acute myeloid leukemia | |
|---|---|
| Other names | Acute myelogenous leukemia, acute nonlymphocytic leukemia (ANLL), acute myeloblastic leukemia, acute granulocytic leukemia[1] |
 | |
| Acute myeloid leukemia. In the cytoplasm of individual cells, you can see characteristic inclusions – Auer rods | |
| Specialty | Hematology, oncology |
| Symptoms | Feeling tired, shortness of breath, easy bruising and bleeding, increased risk of infection[1] |
| Usual onset | All ages, most frequently ~65–75 years old[2] |
| Risk factors | Smoking, previous chemotherapy or radiation therapy, myelodysplastic syndrome, benzene[1] |
| Diagnostic method | Bone marrow aspiration, blood test[3] |
| Treatment | Chemotherapy, radiation therapy, stem cell transplant[1][3] |
| Prognosis | Five-year survival ~29% (US, 2017)[2] |
| Frequency | 1 million (2015)[4] |
| Deaths | 147,100 (2015)[5] |
Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere with normal blood cell production.[1] Symptoms may include feeling tired, shortness of breath, easy bruising and bleeding, and increased risk of infection.[1] Occasionally, spread may occur to the brain, skin, or gums.[1] As an acute leukemia, AML progresses rapidly, and is typically fatal within weeks or months if left untreated.[1]
Risk factors include getting older, being male,[6] smoking, previous chemotherapy or radiation therapy, myelodysplastic syndrome, and exposure to the chemical benzene.[1] The underlying mechanism involves replacement of normal bone marrow with leukemia cells, which results in a drop in red blood cells, platelets, and normal white blood cells.[1] Diagnosis is generally based on bone marrow aspiration and specific blood tests.[3] AML has several subtypes for which treatments and outcomes may vary.[1]
The first-line treatment of AML is usually chemotherapy, with the aim of inducing remission.[1] People may then go on to receive additional chemotherapy, radiation therapy, or a stem cell transplant.[1][3] The specific genetic mutations present within the cancer cells may guide therapy, as well as determine how long that person is likely to survive.[3]
In 2015, AML affected about one million people, and resulted in 147,000 deaths globally.[4][5] It most commonly occurs in older adults.[2] Males are affected more often than females.[2] The five-year survival rate is about 35% in people under 60 years old and 10% in people over 60 years old.[3] Older people whose health is too poor for intensive chemotherapy have a typical survival of five to ten months.[3] It accounts for roughly 1.1% of all cancer cases, and 1.9% of cancer deaths in the United States.[2]
Signs and symptoms
[edit]
Most signs and symptoms of AML are caused by the crowding out in bone marrow of space for normal blood cells to develop.[7] A lack of normal white blood cell production makes people more susceptible to infections.[8] A low red blood cell count (anemia) can cause fatigue, paleness, shortness of breath and palpitations.[8] A lack of platelets can lead to easy bruising, bleeding from the nose (epistaxis), small blood vessels on the skin (petechiae) or gums, or bleeding with minor trauma.[8] Other symptoms may include fever, fatigue worse than what can be attributed to anaemia alone, weight loss and loss of appetite.[8]
Enlargement of the spleen may occur in AML, but it is typically mild and asymptomatic. Lymph node swelling is rare in most types of AML, except for acute myelomonocytic leukemia (AMML).[8] The skin can be involved in the form of leukemia cutis; Sweet's syndrome; or non-specific findings: flat lesions (macules), raised lesion papules, pyoderma gangrenosum and vasculitis.[8]
Some people with AML may experience swelling of the gums because of infiltration of leukemic cells into the gum tissue.[7] Involvement of other parts of the body such as the gastrointestinal tract, respiratory tract and other parts is possible but less common.[8] One area which has particular importance for treatment is whether there is involvement of the meninges around the central nervous system.[8]
Risk factors
[edit]Most cases of AML do not have exposure to any identified risk factors.[9][10] However, a number of risk factors for developing AML have been identified. These include other blood disorders, chemical exposures, ionizing radiation, and genetic risk factors.[9] Where a defined exposure to past chemotherapy, radiotherapy, toxin or hematologic malignancy is known, this is termed secondary AML.[11]
Other blood disorders
[edit]Other blood disorders, particularly myelodysplastic syndrome (MDS) and less commonly myeloproliferative neoplasms (MPN), can evolve into AML;[9] the exact risk depends on the type of MDS/MPN.[12] The presence of asymptomatic clonal hematopoiesis also raises the risk of transformation into AML.[10]
Chemical exposure
[edit]Exposure to chemotherapy, in particular alkylating antineoplastic agents, can increase the risk of subsequently developing AML.[9] Other chemotherapy agents, including fludarabine,[9] and topoisomerase II inhibitors are also associated with the development of AML; most commonly after 4–6 years and 1–3 years respectively.[10] These are often associated with specific chromosomal abnormalities in the leukemic cells.[10]
Other chemical exposures associated with the development of AML include benzene, chloramphenicol and phenylbutazone.[10] The use of Agent Orange, a militarized herbicide used in the Vietnam War has been associated with the risk of AML due to the herbicide regularly having been contaminated by TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin), the most toxic dioxin known.[13]
Radiation
[edit]High amounts of ionizing radiation exposure, such as that used for radiotherapy used to treat some forms of cancer, can increase the risk of AML.[9] People treated with ionizing radiation after treatment for prostate cancer, non-Hodgkin lymphoma, lung cancer, and breast cancer have the highest chance of acquiring AML, but this increased risk returns to the background risk observed in the general population after 12 years.[14] Historically, survivors of the atomic bombings of Hiroshima and Nagasaki had an increased rate of AML,[15] as did radiologists exposed to high levels of X-rays prior to the adoption of modern radiation safety practices.[16]
Genetics
[edit]Most cases of AML arise spontaneously, however there are some genetic mutations associated with an increased risk.[10] Several congenital conditions increase the risk of leukemia; the most common is Down syndrome, with other more rare conditions including Fanconi anemia, Bloom syndrome and ataxia-telangiectasia (all characterised by problems with DNA repair), and Kostmann syndrome.[17]
Other factors
[edit]Being overweight and obese increase the risk of developing AML, as does any amount of active smoking.[11] For reasons that may relate to substance or radiation exposure, certain occupations have a higher rate of AML; particularly work in the nuclear power industry, electronics or computer manufacturing, fishing and animal slaughtering and processing.[11]
Pathophysiology
[edit]
The malignant cell in AML is the myeloblast. In normal development of blood cells (hematopoiesis), the myeloblast is an immature precursor of myeloid white blood cells; a normal myeloblast will mature into a white blood cell such as an eosinophil, basophil, neutrophil or monocyte. In AML, though, a single myeloblast accumulates genetic changes which stop maturation, increase its proliferation, and protect it from programmed cell death (apoptosis).[18] Much of the diversity and heterogeneity of AML is because leukemic transformation can occur at a number of different steps along the differentiation pathway.[18] Genetic abnormalities or the stage at which differentiation was halted form part of modern classification systems.[19]
Specific cytogenetic abnormalities can be found in many people with AML; the types of chromosomal abnormalities often have prognostic significance.[19] The chromosomal translocations encode abnormal fusion proteins, usually transcription factors whose altered properties may cause the "differentiation arrest".[20] For example, in APL, the t(15;17) translocation produces a PML-RARA fusion protein which binds to the retinoic acid receptor element in the promoters of several myeloid-specific genes and inhibits myeloid differentiation.[21]
The clinical signs and symptoms of AML result from the growth of leukemic clone cells, which tends to interfere with the development of normal blood cells in the bone marrow.[22] This leads to neutropenia, anemia, and thrombocytopenia.[22] Other symptoms can arise from the infiltration of malignant cells into parts of the body, such as the gingiva and skin.[22]
Many cells develop mutations in genes that affect epigenetics, such as DNA methylation.[3] When these mutations occur, it is likely in the early stages of AML.[3] Such mutations include in the DNA demethylase TET2 and the metabolic enzymes IDH1 and IDH2,[23] which lead to the generation of a novel oncometabolite, D-2-hydroxyglutarate, which inhibits the activity of epigenetic enzymes such as TET2.[24] Epigenetic mutations may lead to the silencing of tumor suppressor genes and/or the activation of proto-oncogenes.[25]
Diagnosis
[edit]
A complete blood count, which is a blood test, is one of the initial steps in the diagnosis of AML. It may reveal both an excess of white blood cells (leukocytosis) or a decrease (leukopenia), and a low red blood cell count (anemia) and low platelets (thrombocytopenia) can also be commonly seen.[22] A blood film may show leukemic blast cells.[22] Inclusions within the cells called Auer rods, when seen, make the diagnosis highly likely.[22] A definitive diagnosis requires a bone marrow aspiration and biopsy.[18]
Bone marrow is examined under light microscopy, as well as flow cytometry, to diagnose the presence of leukemia, to differentiate AML from other types of leukemia (e.g. acute lymphoblastic leukemia), and to provide information about how mature or immature the affected cells are that can assist in classifying the subtype of disease.[18] A sample of marrow or blood is typically also tested for chromosomal abnormalities by routine cytogenetics or fluorescent in situ hybridization. Genetic studies may also be performed to look for specific mutations in genes such as FLT3, nucleophosmin, and KIT, which may influence the outcome of the disease.[26]
Cytochemical stains on blood and bone marrow smears are helpful in the distinction of AML from ALL, and in subclassification of AML. The combination of a myeloperoxidase or Sudan black stain and a nonspecific esterase stain will provide the desired information in most cases. The myeloperoxidase or Sudan black reactions are most useful in establishing the identity of AML and distinguishing it from ALL. The nonspecific esterase stain is used to identify a monocytic component in AMLs and to distinguish a poorly differentiated monoblastic leukemia from ALL.[27]
The standard classification scheme for AML is the World Health Organization (WHO) system.[28][29] According to the WHO criteria, the diagnosis of AML is established by demonstrating involvement of more than 20% of the blood and/or bone marrow by leukemic myeloblasts, except in three forms of acute myeloid leukemia with recurrent genetic abnormalities: t(8;21), inv(16) or t(16;16), and acute promyelocytic leukemia with PML-RARA, in which the presence of the genetic abnormality is diagnostic irrespective of blast percent.[17] Myeloid sarcoma is also considered a subtype of AML independently of the blast count.[30][31] The older French-American-British (FAB) classification, which is no longer widely used,[29] is a bit more stringent, requiring a blast percentage of at least 30% in bone marrow or peripheral blood for the diagnosis of AML.[32]
Because acute promyelocytic leukemia has the highest curability and requires a unique form of treatment, it is important to quickly establish or exclude the diagnosis of this subtype of leukemia. Fluorescent in situ hybridization performed on blood or bone marrow is often used for this purpose, as it readily identifies the chromosomal translocation [t(15;17)(q22;q12);] that characterizes APL. There is also a need to molecularly detect the presence of PML/RARA fusion protein, which is an oncogenic product of that translocation.[33]
World Health Organization
[edit]The WHO classification of AML attempts to be more clinically useful and to produce more meaningful prognostic information than the FAB criteria. The French-American-British (FAB) classification system is based on morphology to define specific immunotypes. The World Health Organization (WHO) classification reviews chromosome translocations and evidence of dysplasia.[34] SEE French-American-British (FAB) classification system.
Each of the WHO categories contains numerous descriptive subcategories of interest to the hematopathologist and oncologist; however, most of the clinically significant information in the WHO schema is communicated via categorization into one of the subtypes listed below.
The revised fourth edition of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues[35] was released in 2016. This classification, which is based on a combination of genetic and immunophenotypic markers and morphology, defined the subtypes of AML and related neoplasms as shown below.[36][37] In 2022, a new classification was published.[38][39]
| Name | Description | ICD-O |
|---|---|---|
| Acute myeloid leukemia with recurrent genetic abnormalities | Includes:[40]
|
Multiple |
| AML with myelodysplasia-related changes | This category includes people who have had a prior documented myelodysplastic syndrome (MDS) or myeloproliferative disease (MPD) that then has transformed into AML; who have cytogenetic abnormalities characteristic for this type of AML (with previous history of MDS or MPD that has gone unnoticed in the past, but the cytogenetics is still suggestive of MDS/MPD history); or who have AML with morphologic features of myelodysplasia (dysplastic changes in multiple cell lines).[41]
People who have previously received chemotherapy or radiation treatment for a non-MDS/MPD disease, and people who have genetic markers associated with AML with recurrent genetic abnormalities, are excluded from this category. This category of AML occurs most often in elderly people and often has a worse prognosis. Cytogenetic markers for AML with myelodysplasia-related changes include:[42]
|
M9895/3 |
| Therapy-related myeloid neoplasms | This category includes people who have had prior chemotherapy and/or radiation and subsequently develop AML or MDS. These leukemias may be characterized by specific chromosomal abnormalities, and often carry a worse prognosis.[43] | M9920/3 |
| Myeloid sarcoma | This category includes myeloid sarcoma.[44] | |
| Myeloid proliferations related to Down syndrome | This category includes "transient abnormal myelopoiesis" and "myeloid leukemia associated with Down syndrome". In young children, myeloid leukemia associated with Down syndrome has a much better prognosis than other types of childhood AML. The prognosis in older children is similar to conventional AML.[45] | |
| AML not otherwise categorized | Includes subtypes of AML that do not fall into the above categories:[46] | M9861/3 |

Acute leukemias of ambiguous lineage (also known as mixed phenotype or biphenotypic acute leukemia) occur when the leukemic cells can not be classified as either myeloid or lymphoid cells, or where both types of cells are present.[48]
French-American-British
[edit]The French-American-British (FAB) classification system provides terminology that is still sometimes used, and it remains a valuable diagnostic tool in areas without access to genetic testing, this system has largely become obsolete in favor of the WHO classification, which correlates more strongly with treatment outcomes.[29][49]
The FAB system divides AML into eight subtypes, M0 through to M7, based on the type of cell from which the leukemia developed and its degree of maturity. AML of types M0 to M2 may be called acute myeloblastic leukemia. Classification is done by examining the appearance of the malignant cells with light microscopy and/or by using cytogenetics to characterize any underlying chromosomal abnormalities. The subtypes have varying prognoses and responses to therapy.
Six FAB subtypes (M1 through to M6) were initially proposed in 1976,[50] although later revisions added M7 in 1985[51] and M0 in 1987.[52]
| Type | Name | Cytogenetics | Percentage of adults with AML | Immunophenotype[53] | ||||
|---|---|---|---|---|---|---|---|---|
| CD14 | CD15 | CD33 | HLA-DR | Other | ||||
| M0 | acute myeloblastic leukemia, minimally differentiated | 5%[54] | −[55][better source needed] | −[55] | +[55] | +[55] | MPO −[56] | |
| M1 | acute myeloblastic leukemia, without maturation | 15%[54] | − | − | + | + | MPO +[56] | |
| M2 | acute myeloblastic leukemia, with granulocytic maturation | t(8;21)(q22;q22), t(6;9) | 25%[54] | − | + | + | + | |
| M3 | promyelocytic, or acute promyelocytic leukemia (APL) | t(15;17) | 10%[54] | − | + | + | − | |
| M4 | acute myelomonocytic leukemia | inv(16)(p13q22), del(16q) | 20%[54] | <45% | + | + | + | |
| M4eo | myelomonocytic together with bone marrow eosinophilia | inv(16), t(16;16) | 5%[54] | +/−[57] | +[58] | +[58] | CD2+[58] | |
| M5 | acute monoblastic leukemia (M5a) or acute monocytic leukemia (M5b) | del (11q), t(9;11), t(11;19) | 10%[54] | >55% | + | + | + | |
| M6 | acute erythroid leukemias, including erythroleukemia (M6a) and very rare pure erythroid leukemia (M6b) | 5%[54] | − | +/− | +/− | +/− | Glycophorin + | |
| M7 | acute megakaryoblastic leukemia | t(1;22) | 5%[54] | − | − | + | +/− | CD41/CD61+ |
The morphologic subtypes of AML also include rare types not included in the FAB system, such as acute basophilic leukemia, which was proposed as a ninth subtype, M8, in 1999.[59]
Treatment
[edit]First-line treatment of AML consists primarily of chemotherapy, and is divided into two phases: induction and consolidation. The goal of induction therapy is to achieve a complete remission by reducing the number of leukemic cells to an undetectable level; the goal of consolidation therapy is to eliminate any residual undetectable disease and achieve a cure.[60] Hematopoietic stem cell transplantation is usually considered if induction chemotherapy fails or after a person relapses, although transplantation is also sometimes used as front-line therapy for people with high-risk disease. Efforts to use tyrosine kinase inhibitors in AML continue.[61]
Induction
[edit]The goal and purpose of the induction phase is to reach a complete remission. Complete remission does not mean the disease has been cured; rather, it signifies no disease can be detected with available diagnostic methods.[60] All subtypes except acute promyelocytic leukemia are usually given induction chemotherapy with cytarabine and an anthracycline such as daunorubicin or idarubicin.[60] This induction chemotherapy regimen is known as "7+3" (or "3+7"), because the cytarabine is given as a continuous IV infusion for seven consecutive days while the anthracycline is given for three consecutive days as an IV push.[62] Response to this treatment varies with age, with people aged less than 60 years having better remission rates between 60% and 80%, while older people having lower remission rates between 33% and 60%.[60] Because of the toxic effects of therapy and a greater chance of AML resistance to this induction therapy, different treatment, such as that in clinical trials might be offered to people 60–65 years or older.[60]
Acute promyelocytic leukemia is treated with all-trans-retinoic acid (ATRA) and either arsenic trioxide (ATO) monotherapy or an anthracycline.[63] A syndrome similar to disseminated intravascular coagulation can develop during the initial few days of treatment or at the time the leukemia is diagnosed, and treatment can be complicated by a differentiation syndrome characterised by fever, fluid overload and low oxygen levels.[63] Acute promyelocytic leukemia is considered curable.[64] There is insufficient evidence to determine if prescribing ATRA in addition to chemotherapy to adults who have other subtypes of acute myeloid leukaemia is helpful.[65]
Consolidation
[edit]Even after complete remission is achieved, leukemic cells likely remain in numbers too small to be detected with current diagnostic techniques. If no consolidation therapy or further postremission is given, almost all people with AML will eventually relapse.[60]
The specific type of postremission therapy is individualized based on a person's prognostic factors (see above) and general health.[60] For good-prognosis leukemias (i.e. inv(16), t(8;21), and t(15;17)), people will typically undergo an additional three to five courses of intensive chemotherapy, known as consolidation chemotherapy. This generally involves cytarabine, with the doses administered being higher in younger patients, who are less likely to develop toxicity related to this treatment.[60]
Stem cell transplantation
[edit]Stem cell transplantation from a donor, called allogenic stem cell transplantation, is usually pursued if the prognosis is not considered favourable, a person can tolerate a transplant and has a suitable donor.[66] The basis of allogenic stem cell transplantation is on a graft versus leukemia effect whereby graft cells stimulate an immune response against leukemia cells.[66] Unfortunately, this is accompanied by immune responses against other host organs, called a graft versus host disease.[66]
Theoretical therapies have been proposed based on the idea of using stem cell transplantation to replace blood stem cells with genetically modified versions with altered molecular markers, including CD45, which is present on most blood cells.[67] A treatment would then be applied, such as an antibody-drug conjugate targeting the healthy version of the marker, in order to kill all blood cells with unmodified markers, including the original cells and the cancerous ones.[67] Theoretical therapies have also been proposed to use genetic engineering to attach synthetic chimeric antigen receptors to T-cells.[67] These would bind to markers present in high levels in AML cells, which include CD123 and CD135.[67] T-cells could also be modified to target normal CD45 markers, but this requires also modifying the CD-45 of T-cells as well so that they do not target themselves.[67] None of these therapies have entered clinical trials, but some have been tested successfully in mice.[67]
Target therapy
[edit]Target therapy is a type of treatment that uses drugs or other substances to target specific molecules that cancer cells need to survive and spread. Targeted therapies work in different ways to treat cancer. Some stop cancer cells from growing by interrupting signals that cause them to grow and divide, stopping signals that help form blood vessels, delivering cell-killing substances to cancer cells, or starving cancer cells of hormones they need to grow. Other targeted therapies help the immune system kill cancer cells or directly cause cancer cell death. Most targeted therapies are either small-molecule drugs or monoclonal antibodies. Also called molecularly targeted therapy.[68]
Supportive treatment
[edit]Support is necessary throughout treatment because of problems associated with AML and also arising from treatment.[69] Blood transfusions, including of red blood cells and platelets, are necessary to maintain health levels, preventing complications of anemia (from low red blood cells) and bleeding (from low platelets).[69] AML leads to an increased risk of infections, particularly drug-resistant strains of bacteria and fungi.[62] Antibiotics and antifungals can be used both to treat and to prevent these infections, particularly quinolones.[62][70]
Adding aerobic physical exercises to the standard of care may result in little to no difference in the mortality, in the quality of life and in the physical functioning. These exercises may result in a slight reduction in depression. Furthermore, aerobic physical exercises probably reduce fatigue.[71]
Recent research into the role that epigenetic regulators play in hematopoietic malignancies has yielded new insights in the development of targeted epigenetic therapies as a supportive treatment for AML. The FDA has approved certain epigenetic modifying drugs like ivosidenib and enasidenib, which are used in patients that can no longer receive intensive induction chemotherapy; specifically, they are involved in the therapy of IDH1 and IDH2 mutations. Further research must be done to prove the efficacy of epigenetic treatments, but the development of new epigenetic therapies along with immunotherapies holds potential in the future treatment of AML.[72]
In pregnancy
[edit]AML is rare in pregnancy, affecting about 1 in 75,000 to 100,000 pregnant women.[73] It is diagnosed and treated similarly to AML in non pregnancy, with a recommendation that it is treated urgently.[73] However, treatment has significant implications for the pregnancy. First trimester pregnancy is considered unlikely to be viable; pregnancy during weeks 24 – 36 requires consideration of the benefits of chemotherapy to the mother against the risks to the fetus; and there is a recommendation to consider delaying chemotherapy in very late pregnancy (> 36 weeks).[73] Some elements of supportive care, such as which antibiotics to prevent or treat infections, also change in pregnancy.[73]
Medication
[edit]Olutasidenib (Rezlidhia) was approved for medical use in the United States in December 2022.[74]
Prognosis
[edit]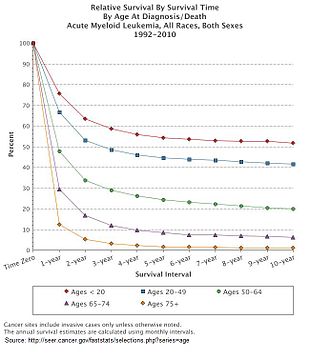

Multiple factors influence prognosis in AML, including the presence of specific mutations, and a person with AML's age. In the United States between 2011 and 2016, the median survival of a person with AML was 8.5 months, with the 5 year survival being 24%.[11] This declines with age, with the poorer prognosis being associated with an age greater than 65 years, and the poorest prognosis seen in those aged 75–84.[11]
As of 2001, cure rates in clinical trials have ranged from 20 to 45%;[75][76] although clinical trials often include only younger people and those able to tolerate aggressive therapies. The overall cure rate for all people with AML (including the elderly and those unable to tolerate aggressive therapy) is likely lower. Cure rates for APL can be as high as 98%.[77]
Subtypes
[edit]Secondary AML has a worse prognosis, as does treatment-related AML arising after chemotherapy for another previous malignancy. Both of these entities are associated with a high rate of unfavorable genetic mutations.[11]
Cytogenetics
[edit]Different genetic mutations are associated with a difference in outcomes. Certain cytogenetic abnormalities are associated with very good outcomes (for example, the (15;17) translocation in APL). About half of people with AML have "normal" cytogenetics; they fall into an intermediate risk group. A number of other cytogenetic abnormalities are known to associate with a poor prognosis and a high risk of relapse after treatment.[78][79][80]
A large number of molecular alterations are under study for their prognostic impact in AML. However, only FLT3-ITD, NPM1, CEBPA and c-KIT are currently included in validated international risk stratification schema. These are expected to increase rapidly in the near future.[3] FLT3 internal tandem duplications (ITDs) have been shown to confer a poorer prognosis in AML with normal cytogenetics. Several FLT3 inhibitors have undergone clinical trials, with mixed results. Two other mutations – NPM1 and biallelic CEBPA are associated with improved outcomes, especially in people with normal cytogenetics and are used in current risk stratification algorithms.[3]
Researchers are investigating the clinical significance of c-KIT mutations in AML. These are prevalent, and potentially clinically relevant because of the availability of tyrosine kinase inhibitors, such as imatinib and sunitinib that can block the activity of c-KIT pharmacologically.[3] It is expected that additional markers (e.g., RUNX1, ASXL1, and TP53) that have consistently been associated with an inferior outcome will soon be included in these recommendations. The prognostic importance of other mutated genes (e.g., DNMT3A, IDH1, IDH2) is less clear.[3][23]
Other prognostic factors
[edit]Elevated lactate dehydrogenase level were also associated with poorer outcomes.[81] Use of tobacco is associated with a person having a poorer prognosis,[11] and people who are married and live together have a better prognosis.[11] People who are treated at place with a higher volume of AML have a better prognosis than those who are treated at those in the lowest quartile.[11] As with most forms of cancer, performance status (i.e. the general physical condition and activity level of the person) plays a major role in prognosis as well.[82]
For people in remission after induction chemotherapy, residual leukemic cells (minimal residual disease) are associated with higher relapse rates and decreased survival.[83] Furthermore, the presence of specific leukemic cells that are capable of initiating a relapse, the leukemia stem cell (a type of cancer stem cell) is associated with impaired survival and higher incidence of relapse.[84]
Epidemiology
[edit]AML is a relatively rare cancer. There were 19,950 new cases in the United States in 2016.[85] In 2018, AML accounted for 1.2% of all cancer deaths in the United States.[10]
The incidence of AML increases with age and varies between countries.[11] The median age when AML is diagnosed ranges between 63 and 71 years in the UK, Canada, Australia and Sweden, compared with 40 to 45 years in India, Brazil and Algeria.[11]
According to 2002 statistics, AML accounts for about 90% of all acute leukemias in adults and is rare in children.[86] Acute leukemias consist of serious medical conditions relating to an original diagnosis of leukemia, where the abnormal blood cells are immature blood cells (blasts). They are mostly classified in terms of myeloid cells or lymphoid cells.[87]
Based on data from United States Cancer Statistics (USCS) Public Use Database for 2001–2017, the 2021 estimate for new cases of AML and acute lymphoblastic leukemia (ALL) are following:[88]
- Total estimated cases for AML: 20,240
- Total estimated cases for ALL: 5,690
Based on these estimates, AML is about 78% of the total cases.
The rate of therapy-related AML (AML caused by previous chemotherapy) is expected to rise with an increase in the use of chemotherapy, an ageing population and more patients surviving their initial chemotherapy treatment; therapy-related disease accounts for just under 10% of all cases of AML.[89] AML is slightly more common in men, with a male-to-female ratio of 1.3:1[90] to 1.4:1.[11] Incidence is also seen to differ by ethnicity, with caucasians having higher recorded incidences and the lowest recorded incidences being in Pacific Islanders and native Alaskans.[11]
In the UK, AML accounts for 31% of all leukemia cases, and around 3,100 people were diagnosed with the disease each year in 2016–2018.[91]
History
[edit]
The first published description of a case of leukemia in medical literature dates to 1827 when French physician Alfred-Armand-Louis-Marie Velpeau described a 63-year-old florist who developed an illness characterized by fever, weakness, urinary stones, and substantial enlargement of the liver and spleen. Velpeau noted the blood of this person had a consistency "like gruel", and speculated the appearance of the blood was due to white corpuscles.[92] In 1845, a series of people who died with enlarged spleens and changes in the "colors and consistencies of their blood" was reported by the Edinburgh-based pathologist J.H. Bennett; he used the term "leucocythemia" to describe this pathological condition.[93]
The term "leukemia" was coined by Rudolf Virchow, the renowned German pathologist, in 1856. As a pioneer in the use of the light microscope in pathology, Virchow was the first to describe the abnormal excess of white blood cells in people with the clinical syndrome described by Velpeau and Bennett. As Virchow was uncertain of the etiology of the white blood cell excess, he used the purely descriptive term "leukemia" (Greek: "white blood") to refer to the condition.[94]
Further advances in the understanding of AML occurred rapidly with the development of new technology. In 1877, Paul Ehrlich developed a technique of staining blood films which allowed him to describe in detail normal and abnormal white blood cells. Wilhelm Ebstein introduced the term "acute leukemia" in 1889 to differentiate rapidly progressive and fatal leukemias from the more indolent chronic leukemias.[95] The term "myeloid" was coined by Franz Ernst Christian Neumann in 1869, as he was the first to recognize white blood cells were made in the bone marrow (Greek: μυєλός, myelos, lit. '(bone) marrow') as opposed to the spleen. The technique of bone marrow examination to diagnose leukemia was first described in 1879 by Mosler.[96] Finally, in 1900, the myeloblast, which is the malignant cell in AML, was characterized by Otto Naegeli, who divided the leukemias into myeloid and lymphocytic.[97][98]
In 2008, AML became the first cancer genome to be fully sequenced. DNA extracted from leukemic cells were compared to unaffected skin.[99] The leukemic cells contained acquired mutations in several genes that had not previously been associated with the disease.
References
[edit]- ^ a b c d e f g h i j k l m "Adult Acute Myeloid Leukemia Treatment". National Cancer Institute. 6 March 2017. Retrieved 19 December 2017.
- ^ a b c d e "Acute Myeloid Leukemia – Cancer Stat Facts". NCI. Retrieved 10 May 2017.
- ^ a b c d e f g h i j k l m Döhner H, Weisdorf DJ, Bloomfield CD (September 2015). "Acute Myeloid Leukemia". The New England Journal of Medicine. 373 (12): 1136–1152. doi:10.1056/NEJMra1406184. PMID 26376137. S2CID 40314260.
- ^ a b Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators) (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ^ a b Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. (GBD 2015 Mortality and Causes of Death Collaborators) (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ^ "Risk Factors for Acute Myeloid Leukemia (AML)". www.cancer.org. Retrieved 5 June 2024.
- ^ a b Hoffbrand AV, Moss PA (2016). "13. Acute myeloid leukaemia". Hoffbrand's essential haematology (Seventh ed.). Chichester, West Sussex. pp. 148–149. ISBN 978-1-118-40867-4. OCLC 909538759.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ a b c d e f g h Kaushansky K, Lichtman MA, Prchal JT, Levi M, Burns LJ, Linch DC, eds. (2021). "Acute myelogenous leukaemia". Williams hematology (Tenth ed.). New York: McGraw Hill. ISBN 978-1-260-46413-9. OCLC 1176325543.
- ^ a b c d e f Khwaja A, Bjorkholm M, Gale RE, Levine RL, Jordan CT, Ehninger G, et al. (March 2016). "Acute myeloid leukaemia". Nature Reviews. Disease Primers. 2 (1): 16010. doi:10.1038/nrdp.2016.10. PMID 27159408. S2CID 4028327.
- ^ a b c d e f g Harrison's 2018, p. 739.
- ^ a b c d e f g h i j k l m Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM (July 2019). "Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges". Blood Reviews. 36: 70–87. doi:10.1016/j.blre.2019.04.005. PMID 31101526. S2CID 155630692.
- ^ Sanz GF, Sanz MA, Vallespí T, Cañizo MC, Torrabadella M, García S, et al. (July 1989). "Two regression models and a scoring system for predicting survival and planning treatment in myelodysplastic syndromes: a multivariate analysis of prognostic factors in 370 patients". Blood. 74 (1): 395–408. doi:10.1182/blood.V74.1.395.395. PMID 2752119.
- ^ Shallis RM, Gore SD. Agent Orange and dioxin-induced myeloid leukemia: a weaponized vehicle of leukemogenesis. Leuk Lymphoma. 2022 Jul;63(7):1534–1543. doi: 10.1080/10428194.2022.2034156. Epub 2022 Feb 2. PMID 35105250.
- ^ Radivoyevitch T, Sachs RK, Gale RP, Molenaar RJ, Brenner DJ, Hill BT, et al. (February 2016). "Defining AML and MDS second cancer risk dynamics after diagnoses of first cancers treated or not with radiation". Leukemia. 30 (2): 285–294. doi:10.1038/leu.2015.258. PMID 26460209. S2CID 22100511.
- ^ Bizzozero OJ, Johnson KG, Ciocco A (May 1966). "Radiation-related leukemia in Hiroshima and Nagasaki, 1946–1964. I. Distribution, incidence and appearance time". The New England Journal of Medicine. 274 (20): 1095–1101. doi:10.1056/NEJM196605192742001. PMID 5932020.
- ^ Yoshinaga S, Mabuchi K, Sigurdson AJ, Doody MM, Ron E (November 2004). "Cancer risks among radiologists and radiologic technologists: review of epidemiologic studies". Radiology. 233 (2): 313–321. doi:10.1148/radiol.2332031119. PMID 15375227. S2CID 20643232.
- ^ a b Harrison's 2018, p. 740.
- ^ a b c d Hoffbrand's 2016, pp. 146–7.
- ^ a b Harrison's 2018, p. 742.
- ^ Greer JP, Foerster J, Lukens JN, Rogers GM, Paraskevas F, Glader BE, eds. (2004). Wintrobe's Clinical Hematology (11th ed.). Philadelphia: Lippincott, Williams, and Wilkins. pp. 2045–2062. ISBN 978-0-7817-3650-3.
- ^ Harrison's 2018, p. 741.
- ^ a b c d e f Harrison's 2018, p. 743.
- ^ a b Molenaar RJ, Thota S, Nagata Y, Patel B, Clemente M, Przychodzen B, et al. (November 2015). "Clinical and biological implications of ancestral and non-ancestral IDH1 and IDH2 mutations in myeloid neoplasms". Leukemia. 29 (11): 2134–2142. doi:10.1038/leu.2015.91. PMC 5821256. PMID 25836588.
- ^ Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, Bleeker FE (December 2014). "The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation". Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1846 (2): 326–341. doi:10.1016/j.bbcan.2014.05.004. PMID 24880135.
- ^ Sharma S, Kelly TK, Jones PA (January 2010). "Epigenetics in cancer". Carcinogenesis. 31 (1): 27–36. doi:10.1093/carcin/bgp220. PMC 2802667. PMID 19752007.
- ^ Baldus CD, Mrózek K, Marcucci G, Bloomfield CD (June 2007). "Clinical outcome of de novo acute myeloid leukaemia patients with normal cytogenetics is affected by molecular genetic alterations: a concise review". British Journal of Haematology. 137 (5): 387–400. doi:10.1111/j.1365-2141.2007.06566.x. PMID 17488484. S2CID 30419482.
- ^ Vardiman JW, Harris NL, Brunning RD (October 2002). "The World Health Organization (WHO) classification of the myeloid neoplasms". Blood. 100 (7): 2292–2302. doi:10.1182/blood-2002-04-1199. PMID 12239137. S2CID 9413654.
- ^ Greer JP, Arber DA, Glader BE, List AF, Means RM, Rodgers GM (2018). "Chapter 74: Diagnosis and Classification of the Acute Leukemias and Myelodysplastic Syndromes". Wintrobe's Clinical Hematology (14th ed.). Wolters Kluwer Health. ISBN 978-1-4963-6713-6.
- ^ a b c Bain BJ (2015). Blood Cells: A Practical Guide (5 ed.). John Wiley & Sons. p. 432. ISBN 978-1-118-81733-9.
- ^ Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, et al. (2017). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer. p. 25. ISBN 978-92-832-4494-3.
- ^ Foucar K. "Bone Marrow Pathology" (PDF) (3rd ed.). ASCP. Archived from the original (PDF) on 19 March 2013. Retrieved 18 March 2016.
- ^ Amin HM, Yang Y, Shen Y, Estey EH, Giles FJ, Pierce SA, et al. (September 2005). "Having a higher blast percentage in circulation than bone marrow: clinical implications in myelodysplastic syndrome and acute lymphoid and myeloid leukemias". Leukemia. 19 (9): 1567–1572. doi:10.1038/sj.leu.2403876. PMID 16049515. S2CID 11711013.
- ^ Grimwade D, Howe K, Langabeer S, Davies L, Oliver F, Walker H, et al. (September 1996). "Establishing the presence of the t(15;17) in suspected acute promyelocytic leukaemia: cytogenetic, molecular and PML immunofluorescence assessment of patients entered into the M.R.C. ATRA trial. M.R.C. Adult Leukaemia Working Party". British Journal of Haematology. 94 (3): 557–573. doi:10.1046/j.1365-2141.1996.d01-1004.x. PMID 8790159. S2CID 8271000.
- ^ "Acute Myeloid Leukemia (AML) Subtypes and Prognostic Factors".
- ^ Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW (2016). "The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia". Blood. 127 (20): 2391–2705. doi:10.1182/blood-2016-03-643544. PMID 27069254. S2CID 18338178.
- ^ Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. (May 2016). "The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia". Blood. 127 (20): 2391–2405. doi:10.1182/blood-2016-03-643544. PMID 27069254. S2CID 18338178.
- ^ Wintrobe's 2018, Chapter 74, sec. "Acute myeloid leukemia".
- ^ Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. (July 2022). "The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms". Leukemia. 36 (7): 1703–1719. doi:10.1038/s41375-022-01613-1. PMC 9252913. PMID 35732831.
- ^ Molica M, Perrone S (November 2022). "Molecular targets for the treatment of AML in the forthcoming 5th World Health Organization Classification of Haematolymphoid Tumours". Expert Review of Hematology. 15 (11): 973–986. doi:10.1080/17474086.2022.2140137. PMID 36271671. S2CID 253063457.
- ^ Campo 2017, p. 130–145.
- ^ Campo 2017, p. 150.
- ^ Campo 2017, p. 150–152.
- ^ Campo 2017, p. 153–155.
- ^ Campo 2017, p. 167.
- ^ Campo 2017, p. 169–171.
- ^ Campo 2017, p. 156–166.
- ^ Estey EH (October 2018). "Acute myeloid leukemia: 2019 update on risk-stratification and management". American Journal of Hematology. 93 (10): 1267–1291. doi:10.1002/ajh.25214. PMID 30328165. S2CID 53523035.
- ^ Wintrobe's 2018, Chapter 74, sec. "Acute leukemias of ambiguous lineage".
- ^ Wintrobe's 2018, Chapter 74, sec. "Introduction".
- ^ Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C (August 1976). "Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group". British Journal of Haematology. 33 (4): 451–458. doi:10.1111/j.1365-2141.1976.tb03563.x. PMID 188440. S2CID 9985915.
- ^ Bloomfield CD, Brunning RD (September 1985). "FAB M7: acute megakaryoblastic leukemia--beyond morphology". Annals of Internal Medicine. 103 (3): 450–452. doi:10.7326/0003-4819-103-3-450. PMID 4040724.
- ^ Lee EJ, Pollak A, Leavitt RD, Testa JR, Schiffer CA (November 1987). "Minimally differentiated acute nonlymphocytic leukemia: a distinct entity". Blood. 70 (5): 1400–1406. doi:10.1182/blood.v70.5.1400.bloodjournal7051400 (inactive 2 December 2024). PMID 3663939.
{{cite journal}}: CS1 maint: DOI inactive as of December 2024 (link) - ^ Unless otherwise specified in boxes, reference is: Page 97 in: Sun T (2008). Flow cytometry and immunohistochemistry for hematologic neoplasms. Philadelphia: Lippincott Williams & Wilkins. ISBN 978-0-7817-8400-9. OCLC 85862340.
- ^ a b c d e f g h i Seiter K, Jules EH (20 May 2011). "Acute Myeloid Leukemia Staging". Retrieved 26 August 2011.
- ^ a b c d Mihova D. "Leukemia acute – Acute myeloid leukemia with minimal differentiation (FAB AML M0)". Pathology Outlines. Topic Completed: 1 March 2013. Minor changes: 19 November 2019
- ^ a b Salem DA, Abd El-Aziz SM (June 2012). "Flowcytometric immunophenotypic profile of acute leukemia: mansoura experience". Indian Journal of Hematology & Blood Transfusion. 28 (2): 89–96. doi:10.1007/s12288-011-0110-2. PMC 3332273. PMID 23730015.
- ^ Partial expression: Adriaansen HJ, te Boekhorst PA, Hagemeijer AM, van der Schoot CE, Delwel HR, van Dongen JJ (June 1993). "Acute myeloid leukemia M4 with bone marrow eosinophilia (M4Eo) and inv(16)(p13q22) exhibits a specific immunophenotype with CD2 expression". Blood. 81 (11): 3043–3051. doi:10.1182/blood.V81.11.3043.bloodjournal81113043 (inactive 2 December 2024). PMID 8098967.
{{cite journal}}: CS1 maint: DOI inactive as of December 2024 (link) - ^ a b c Page 99 in: Sun T (2009). Atlas of hematologic neoplasms. Dordrecht New York: Springer. ISBN 978-0-387-89848-3. OCLC 432709321.
- ^ Duchayne E, Demur C, Rubie H, Robert A, Dastugue N (January 1999). "Diagnosis of acute basophilic leukemia". Leukemia & Lymphoma. 32 (3–4): 269–278. doi:10.3109/10428199909167387. PMID 10037024.
- ^ a b c d e f g h Harrison's 2018, p. 743-5.
- ^ Kayser S, Levis MJ (February 2014). "FLT3 tyrosine kinase inhibitors in acute myeloid leukemia: clinical implications and limitations". Leukemia & Lymphoma. 55 (2): 243–255. doi:10.3109/10428194.2013.800198. PMC 4333682. PMID 23631653.
- ^ a b c Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. (January 2017). "Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel". Blood. 129 (4): 424–447. doi:10.1182/blood-2016-08-733196. PMC 5291965. PMID 27895058.
- ^ a b Hoffbrand's 2016, pp. 152–3.
- ^ Harrison's 2018, p. 748.
- ^ Küley-Bagheri Y, Kreuzer KA, Monsef I, Lübbert M, Skoetz N, et al. (Cochrane Haematological Malignancies Group) (August 2018). "Effects of all-trans retinoic acid (ATRA) in addition to chemotherapy for adults with acute myeloid leukaemia (AML) (non-acute promyelocytic leukaemia (non-APL))". The Cochrane Database of Systematic Reviews. 2018 (8): CD011960. doi:10.1002/14651858.CD011960.pub2. PMC 6513628. PMID 30080246.
- ^ a b c Harrison's 2018, p. 745.
- ^ a b c d e f Mole B (15 July 2024). "Genetic cloaking of healthy cells opens door to universal blood cancer therapy". Ars Technica. Retrieved 15 July 2024.
- ^ "Acute Myeloid Leukemia Treatment – NCI". www.cancer.gov. 7 June 2023. Retrieved 14 June 2023.
- ^ a b Harrison's 2018, p. 747.
- ^ Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. (January 2010). "Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet". Blood. 115 (3): 453–474. doi:10.1182/blood-2009-07-235358. PMID 19880497. S2CID 1088077.
- ^ Knips L, Bergenthal N, Streckmann F, Monsef I, Elter T, Skoetz N, et al. (Cochrane Haematological Malignancies Group) (January 2019). "Aerobic physical exercise for adult patients with haematological malignancies". The Cochrane Database of Systematic Reviews. 1 (1): CD009075. doi:10.1002/14651858.CD009075.pub3. PMC 6354325. PMID 30702150.
- ^ Verma S, Dhanda H, Singh A, Rishi B, Tanwar P, Chaudhry S, et al. (October 2021). "Systematic review of epigenetic targets in acute myeloid leukemia". American Journal of Blood Research. 11 (5): 458–471. PMC 8610793. PMID 34824880.
- ^ a b c d Ali S, Jones GL, Culligan DJ, Marsden PJ, Russell N, Embleton ND, Craddock C (August 2015). "Guidelines for the diagnosis and management of acute myeloid leukaemia in pregnancy". British Journal of Haematology. 170 (4): 487–495. doi:10.1111/bjh.13554. PMID 26081614. S2CID 11298224.
- ^ "Rigel Announces U.S. FDA Approval of Rezlidhia (olutasidenib) for the Treatment of Adult Patients with Relapsed or Refractory Acute Myeloid Leukemia with a Susceptible IDH1 Mutation" (Press release). Rigel Pharmaceuticals. 1 December 2022. Retrieved 2 December 2022 – via PR Newswire.
- ^ Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta E, et al. (December 1998). "Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission". The New England Journal of Medicine. 339 (23): 1649–1656. doi:10.1056/NEJM199812033392301. PMID 9834301.
- ^ Matthews JP, Bishop JF, Young GA, Juneja SK, Lowenthal RM, Garson OM, et al. (June 2001). "Patterns of failure with increasing intensification of induction chemotherapy for acute myeloid leukaemia". British Journal of Haematology. 113 (3): 727–736. doi:10.1046/j.1365-2141.2001.02756.x. PMID 11380464. S2CID 34233226.
- ^ Sanz MA, Lo Coco F, Martín G, Avvisati G, Rayón C, Barbui T, et al. (August 2000). "Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups". Blood. 96 (4): 1247–1253. PMID 10942364. Archived from the original on 27 May 2010. Retrieved 17 March 2008.
- ^ Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, et al. (October 1999). "A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties". British Journal of Haematology. 107 (1): 69–79. doi:10.1046/j.1365-2141.1999.01684.x. PMID 10520026. S2CID 27266593.
- ^ Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. (December 2000). "Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study". Blood. 96 (13): 4075–4083. doi:10.1182/blood.V96.13.4075. PMID 11110676.
- ^ Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. (December 2002). "Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461)". Blood. 100 (13): 4325–4336. doi:10.1182/blood-2002-03-0772. PMID 12393746. S2CID 16003833.
- ^ Haferlach T, Schoch C, Löffler H, Gassmann W, Kern W, Schnittger S, et al. (January 2003). "Morphologic dysplasia in de novo acute myeloid leukemia (AML) is related to unfavorable cytogenetics but has no independent prognostic relevance under the conditions of intensive induction therapy: results of a multiparameter analysis from the German AML Cooperative Group studies". Journal of Clinical Oncology. 21 (2): 256–265. doi:10.1200/JCO.2003.08.005. PMID 12525517.
- ^ "Acute Myeloid Leukemia (AML)". Yale Medicine. Retrieved 21 June 2024.
- ^ Jongen-Lavrencic M, Grob T, Hanekamp D, Kavelaars FG, al Hinai A, Zeilemaker A, Erpelinck-Verschueren CA, Gradowska PL, Meijer R, Cloos J, Biemond BJ, Graux C, van Marwijk Kooy M, Manz MG, Pabst T (29 March 2018). "Molecular Minimal Residual Disease in Acute Myeloid Leukemia". New England Journal of Medicine. 378 (13): 1189–1199. doi:10.1056/NEJMoa1716863. ISSN 0028-4793. PMID 29601269.
- ^ Reuvekamp T, Bachas C, Cloos J (24 July 2024). "Immunophenotypic features of early haematopoietic and leukaemia stem cels". International Journal of Laboratory Hematology. 46 (5): 795–808. doi:10.1111/ijlh.14348. PMID 39045906.
- ^ Jameson JL, Kasper DL, Fauci AS, Hauser SL, Longo DL, Loscalzo J (2018). Harrison's Principles of Internal Medicine (20 ed.). McGraw-Hill Professional. ISBN 9781259644030.
- ^ Jemal A, Thomas A, Murray T, Thun M (2002). "Cancer statistics, 2002". CA. 52 (1): 23–47. doi:10.3322/canjclin.52.1.23. PMID 11814064. S2CID 5659023.
- ^ "Leukemia – Symptoms and causes". Mayo Clinic. Retrieved 21 June 2024.
- ^ "Facts 2020–2021" (PDF).
- ^ Strickland SA, Vey N (March 2022). "Diagnosis and treatment of therapy-related acute myeloid leukemia". Critical Reviews in Oncology/Hematology. 171: 103607. doi:10.1016/j.critrevonc.2022.103607. PMID 35101585. S2CID 246443548.
- ^ Greenlee RT, Hill-Harmon MB, Murray T, Thun M (2001). "Cancer statistics, 2001". CA. 51 (1): 15–36. doi:10.3322/canjclin.51.1.15. PMID 11577478. S2CID 22441565.
- ^ "Acute myeloid leukaemia (AML) statistics". Cancer Research UK. 14 May 2015. Retrieved 3 April 2022.
- ^ Hoffman R (2005). Hematology: Basic Principles and Practice (4th ed.). St. Louis, Mo.: Elsevier Churchill Livingstone. p. 1071. ISBN 978-0-443-06629-0.
- ^ Bennett JH (1845). "Two cases of hypertrophy of the spleen and liver, in which death took place from suppuration of blood". Edinburgh Med Surg J. 64: 413.
- ^ Virchow R (1856). "Die Leukämie". In Virchow R (ed.). Gesammelte Abhandlungen zur Wissenschaftlichen Medizin (in German). Frankfurt: Meidinger. p. 190.
- ^ Ebstein W (1889). "Über die acute Leukämie und Pseudoleukämie". Deutsch Arch Klin Med. 44: 343.
- ^ Mosler F (1876). "Klinische Symptome und Therapie der medullären Leukämie". Berl Klin Wochenschr. 13: 702.
- ^ Naegeli O (1900). "Über rothes Knochenmark und Myeloblasten". Deutsche Medizinische Wochenschrift. 26 (18): 287–290. doi:10.1055/s-0029-1203820. S2CID 71572772.
- ^ Wang ZY (2003). "Ham-Wasserman lecture: treatment of acute leukemia by inducing differentiation and apoptosis". Hematology. American Society of Hematology. Education Program. 2003 (1): 1–13. doi:10.1182/asheducation-2003.1.1 (inactive 2 December 2024). PMID 14633774. S2CID 2788589.
{{cite journal}}: CS1 maint: DOI inactive as of December 2024 (link) - ^ Ley TJ, Mardis ER, Ding L, Fulton B, McLellan MD, Chen K, et al. (November 2008). "DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome". Nature. 456 (7218): 66–72. Bibcode:2008Natur.456...66L. doi:10.1038/nature07485. PMC 2603574. PMID 18987736.
