Ctenophora
| Comb jellies | |
|---|---|
| |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Ctenophora Eschscholtz, 1829 |
| Classes | |
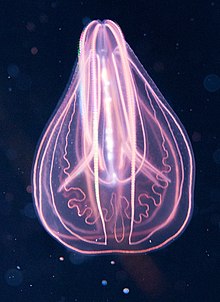
Ctenophora (/təˈnɒfərə/ tə-NOF-ər-ə; sg.: ctenophore /ˈtɛnəfɔːr, ˈtiːnə-/ TEN-ə-for, TEE-nə-; from Ancient Greek κτείς (kteis) 'comb' and φέρω (pherō) 'to carry')[6] comprise a phylum of marine invertebrates, commonly known as comb jellies, that inhabit sea waters worldwide. They are notable for the groups of cilia they use for swimming (commonly referred to as "combs"), and they are the largest animals to swim with the help of cilia.
Depending on the species, adult ctenophores range from a few millimeters to 1.5 m (5 ft) in size. Only 186 living species are currently recognised.[7]
Their bodies consist of a mass of jelly, with a layer two cells thick on the outside, and another lining the internal cavity. The phylum has a wide range of body forms, including the egg-shaped cydippids with a pair of retractable tentacles that capture prey, the flat, generally combless platyctenids, and the large-mouthed beroids, which prey on other ctenophores.
Almost all ctenophores function as predators, taking prey ranging from microscopic larvae and rotifers to the adults of small crustaceans; the exceptions are juveniles of two species, which live as parasites on the salps on which adults of their species feed.
Despite their soft, gelatinous bodies, fossils thought to represent ctenophores appear in Lagerstätten dating as far back as the early Cambrian, about 525 million years ago. The position of the ctenophores in the "tree of life" has long been debated in molecular phylogenetics studies. Biologists proposed that ctenophores constitute the second-earliest branching animal lineage, with sponges being the sister-group to all other multicellular animals (Porifera sister hypothesis).[8] Other biologists contend that ctenophores emerged earlier than sponges (Ctenophora sister hypothesis), which themselves appeared before the split between cnidarians and bilaterians.[9][10] Pisani et al. reanalyzed the data and suggested that the computer algorithms used for analysis were misled by the presence of specific ctenophore genes that were markedly different from those of other species.[11][12][page needed] Follow up analysis by Whelan et al. (2017)[13] yielded further support for the 'Ctenophora sister' hypothesis; the issue remains a matter of taxonomic dispute.[14][15] Schultz et al. (2023) found irreversible changes in synteny in the sister of the Ctenophora, the Myriazoa, consisting of the rest of the animals.[16]
Distinguishing features
[edit]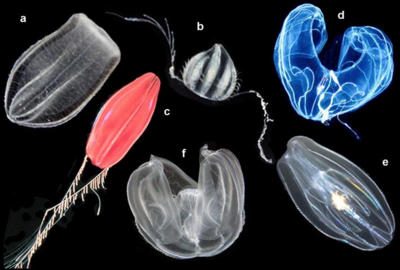
a Beroe ovata, b unidentified cydippid, c "Tortugas red" cydippid,
d Bathocyroe fosteri, e Mnemiopsis leidyi, and f Ocyropsis sp.[17]
Among animal phyla, the Ctenophores are more complex than sponges, about as complex as cnidarians (jellyfish, sea anemones, etc.), and less complex than bilaterians (which include almost all other animals). Unlike sponges, both ctenophores and cnidarians have:
- cells bound by inter-cell connections and
- carpet-like basement membranes;
- muscles;
- nervous systems; and
- sensory organs (in some, not all).
Ctenophores are distinguished from all other animals by having colloblasts, which are sticky and adhere to prey, although a few ctenophore species lack them.[18][19]
Like cnidarians, ctenophores have two main layers of cells that sandwich a middle layer of jelly-like material, which is called the mesoglea in cnidarians and ctenophores; more complex animals have three main cell layers and no intermediate jelly-like layer. Hence ctenophores and cnidarians have traditionally been labelled diploblastic.[18][20] Both ctenophores and cnidarians have a type of muscle that, in more complex animals, arises from the middle cell layer,[21] and as a result some recent text books classify ctenophores as triploblastic,[22] while others still regard them as diploblastic.[18] The comb jellies have more than 80 different cell types, exceeding the numbers from other groups like placozoans, sponges, cnidarians, and some deep-branching bilaterians.[23]
Ranging from about 1 millimeter (0.04 in) to 1.5 meters (5 ft) in size,[22][24] ctenophores are the largest non-colonial animals that use cilia as their main method of locomotion.[22] Most species have eight strips, called comb rows, that run the length of their bodies and bear comb-like bands of cilia, called "ctenes", stacked along the comb rows so that when the cilia beat, those of each comb touch the comb below.[22] The name "ctenophora" means "comb-bearing", from the Greek κτείς (stem-form κτεν-) meaning "comb" and the Greek suffix -φορος meaning "carrying".[25]
| Sponges[26][27] | Cnidarians[18][20][28] | Ctenophores[18][22] | Bilateria[18] | |
|---|---|---|---|---|
| Cnidocytes | No | Yes | Only in some species (obtained from ingested cnidarians) | |
| microRNA | Yes | Yes | No | Yes |
| Hox genes | No | Yes | No | Yes |
| Colloblasts | No | In most species[19] | No | |
| Digestive and circulatory organs | No | Yes | ||
| Anal pores | No | Yes | Mostly Yes | |
| Number of main cell layers | Two, with jelly-like layer between them | Debate about whether two[18] or three[21][22] | Three | |
| Cells in each layer bound together | No, except that Homoscleromorpha have basement membranes[29] | Yes: Inter-cell connections; basement membranes | ||
| Sensory organs | No | Yes | ||
| Eyes (e.g. ocelli) |
No | Yes | No | Yes |
| Apical organ | No | Yes | Yes | In species with primary ciliated larvae |
| Cell abundance in middle "jelly" layer |
Many | Few | [not applicable] | |
| Outer layer cells can move inwards and change functions |
Yes | No | ||
| Nervous system | No | Yes, simple | Simple to complex | |
| Muscles | None | Mostly epitheliomuscular | Mostly myoepithelial | Mostly myocytes |
Description
[edit]For a phylum with relatively few species, ctenophores have a wide range of body plans.[22] Coastal species need to be tough enough to withstand waves and swirling sediment particles, while some oceanic species are so fragile that it is very difficult to capture them intact for study.[19] In addition, oceanic species do not preserve well,[19] and are known mainly from photographs and from observers' notes.[30] Hence most attention has until recently concentrated on three coastal genera – Pleurobrachia, Beroe and Mnemiopsis.[19][31] At least two textbooks base their descriptions of ctenophores on the cydippid Pleurobrachia.[18][22]
Since the body of many species is almost radially symmetrical, the main axis is oral to aboral (from the mouth to the opposite end). However, since only two of the canals near the statocyst terminate in anal pores, ctenophores have no mirror-symmetry, although many have rotational symmetry. In other words, if the animal rotates in a half-circle it looks the same as when it started.[32]
Common features
[edit]The Ctenophore phylum has a wide range of body forms, including the flattened, deep-sea platyctenids, in which the adults of most species lack combs, and the coastal beroids, which lack tentacles and prey on other ctenophores by using huge mouths armed with groups of large, stiffened cilia that act as teeth.
Body layers
[edit]
Like those of cnidarians, (jellyfish, sea anemones, etc.), ctenophores' bodies consist of a relatively thick, jelly-like mesoglea sandwiched between two epithelia, layers of cells bound by inter-cell connections and by a fibrous basement membrane that they secrete.[18][22] The epithelia of ctenophores have two layers of cells rather than one, and some of the cells in the upper layer have several cilia per cell.[22]
The outer layer of the epidermis (outer skin) consists of: sensory cells; cells that secrete mucus, which protects the body; and interstitial cells, which can transform into other types of cell. In specialized parts of the body, the outer layer also contains colloblasts, found along the surface of tentacles and used in capturing prey, or cells bearing multiple large cilia, for locomotion. The inner layer of the epidermis contains a nerve net, and myoepithelial cells that act as muscles.[22]
The internal cavity forms: a mouth that can usually be closed by muscles; a pharynx ("throat"); a wider area in the center that acts as a stomach; and a system of internal canals. These branch through the mesoglea to the most active parts of the animal: the mouth and pharynx; the roots of the tentacles, if present; all along the underside of each comb row; and four branches around the sensory complex at the far end from the mouth – two of these four branches terminate in anal pores. The inner surface of the cavity is lined with an epithelium, the gastrodermis. The mouth and pharynx have both cilia and well-developed muscles. In other parts of the canal system, the gastrodermis is different on the sides nearest to and furthest from the organ that it supplies. The nearer side is composed of tall nutritive cells that store nutrients in vacuoles (internal compartments), germ cells that produce eggs or sperm, and photocytes that produce bioluminescence. The side furthest from the organ is covered with ciliated cells that circulate water through the canals, punctuated by ciliary rosettes, pores that are surrounded by double whorls of cilia and connect to the mesoglea.[22]
Feeding, excretion and respiration
[edit]When prey is swallowed, it is liquefied in the pharynx by enzymes and by muscular contractions of the pharynx. The resulting slurry is wafted through the canal system by the beating of the cilia, and digested by the nutritive cells. The ciliary rosettes in the canals may help to transport nutrients to muscles in the mesoglea. The anal pores may eject unwanted small particles, but most unwanted matter is regurgitated via the mouth.[22]
Little is known about how ctenophores get rid of waste products produced by the cells. The ciliary rosettes in the gastrodermis may help to remove wastes from the mesoglea, and may also help to adjust the animal's buoyancy by pumping water into or out of the mesoglea.[22]
Locomotion
[edit]The outer surface bears usually eight comb rows, called swimming-plates, which are used for swimming. The rows are oriented to run from near the mouth (the "oral pole") to the opposite end (the "aboral pole"), and are spaced more or less evenly around the body,[18] although spacing patterns vary by species and in most species the comb rows extend only part of the distance from the aboral pole towards the mouth. The "combs" (also called "ctenes" or "comb plates") run across each row, and each consists of thousands of unusually long cilia, up to 2 millimeters (0.08 in). Unlike conventional cilia and flagella, which has a filament structure arranged in a 9 + 2 pattern, these cilia are arranged in a 9 + 3 pattern, where the extra compact filament is suspected to have a supporting function.[33] These normally beat so that the propulsion stroke is away from the mouth, although they can also reverse direction. Hence ctenophores usually swim in the direction in which the mouth is eating, unlike jellyfish.[22] When trying to escape predators, one species can accelerate to six times its normal speed;[34] some other species reverse direction as part of their escape behavior, by reversing the power stroke of the comb plate cilia.
It is uncertain how ctenophores control their buoyancy, but experiments have shown that some species rely on osmotic pressure to adapt to the water of different densities.[35] Their body fluids are normally as concentrated as seawater. If they enter less dense brackish water, the ciliary rosettes in the body cavity may pump this into the mesoglea to increase its bulk and decrease its density, to avoid sinking. Conversely, if they move from brackish to full-strength seawater, the rosettes may pump water out of the mesoglea to reduce its volume and increase its density.[22]
Nervous system and senses
[edit]Ctenophores have no brain or central nervous system, but instead have a subepidermal nerve net (rather like a cobweb) that forms a ring round the mouth and is densest near structures such as the comb rows, pharynx, tentacles (if present) and the sensory complex furthest from the mouth.[22] The communication between nerve cells make use of two different methods; some of the neurons are found to have synaptic connections, but the neurons in the nerve net are highly distinctive by being fused into a syncytium, rather than being connected by synapses. Some animals outside ctenophores also have fused nerve cells, but never to such a degree that they form a whole nerve net.[36][37][38] Fossils shows that Cambrian species had a more complex nervous system, with long nerves which connected with a ring around the mouth. The only known ctenophores with long nerves today is Euplokamis in the order Cydippida.[39] Their nerve cells arise from the same progenitor cells as the colloblasts.[40]
In addition there is a less organized mesogleal nerve net consisting of single neurites. The largest single sensory feature is the aboral organ (at the opposite end from the mouth), which is underlined with its own nerve net.[41] This organ's main component is a statocyst, a balance sensor consisting of a statolith, a tiny grain of calcium carbonate, supported on four bundles of cilia, called "balancers", that sense its orientation. The statocyst is protected by a transparent dome made of long, immobile cilia. A ctenophore does not automatically try to keep the statolith resting equally on all the balancers. Instead, its response is determined by the animal's "mood", in other words, the overall state of the nervous system. For example, if a ctenophore with trailing tentacles captures prey, it will often put some comb rows into reverse, spinning the mouth towards the prey.[22]
Research supports the hypothesis that the ciliated larvae in cnidarians and bilaterians share an ancient and common origin.[42] The larvae's apical organ is involved in the formation of the nervous system.[43] The aboral organ of comb jellies is not homologous with the apical organ in other animals, and the formation of their nervous system has therefore a different embryonic origin.[44]
Ctenophore nerve cells and nervous system have different biochemistry as compared to other animals. For instance, they lack the genes and enzymes required to manufacture neurotransmitters like serotonin, dopamine, nitric oxide, octopamine, noradrenaline, and others, otherwise seen in all other animals with a nervous system, with the genes coding for the receptors for each of these neurotransmitters missing.[45] Monofunctional catalase (CAT), one of the three major families of antioxidant enzymes that target hydrogen peroxide, an important signaling molecule for synaptic and neuronal activity, is also absent, most likely due to gene loss.[46] They have been found to use L-glutamate as a neurotransmitter, and have an unusually high variety of ionotropic glutamate receptors and genes for glutamate synthesis and transport compared to other metazoans.[47] The genomic content of the nervous system genes is the smallest known of any animal, and could represent the minimum genetic requirements for a functional nervous system.[48] The fact that portions of the nervous system feature directly fused neurons, without synapses, suggests that ctenophores might form a sister group to other metazoans, having developed a nervous system independently.[38] If ctenophores are the sister group to all other metazoans, nervous systems may have either been lost in sponges and placozoans, or arisen more than once among metazoans.[49]
Cydippids
[edit]
Cydippid ctenophores have bodies that are more or less rounded, sometimes nearly spherical and other times more cylindrical or egg-shaped; the common coastal "sea gooseberry", Pleurobrachia, sometimes has an egg-shaped body with the mouth at the narrow end,[22] although some individuals are more uniformly round. From opposite sides of the body extends a pair of long, slender tentacles, each housed in a sheath into which it can be withdrawn.[18] Some species of cydippids have bodies that are flattened to various extents so that they are wider in the plane of the tentacles.[22]
The tentacles of cydippid ctenophores are typically fringed with tentilla ("little tentacles"), although a few genera have simple tentacles without these side branches. The tentacles and tentilla are densely covered with microscopic colloblasts that capture prey by sticking to it. Colloblasts are specialized mushroom-shaped cells in the outer layer of the epidermis, and have three main components: a domed head with vesicles (chambers) that contain adhesive; a stalk that anchors the cell in the lower layer of the epidermis or in the mesoglea; and a spiral thread that coils round the stalk and is attached to the head and to the root of the stalk. The function of the spiral thread is uncertain, but it may absorb stress when prey tries to escape, and thus prevent the colloblast from being torn apart.[22] One species, Minictena luteola, which only measure 1.5mm in diameter, have five different types of colloblast cells.[50][51]
In addition to colloblasts, members of the genus Haeckelia, which feed mainly on jellyfish, incorporate their victims' stinging nematocytes into their own tentacles – some cnidaria-eating nudibranchs similarly incorporate nematocytes into their bodies for defense.[52] The tentilla of Euplokamis differ significantly from those of other cydippids: they contain striated muscle, a cell type otherwise unknown in the phylum Ctenophora; and they are coiled when relaxed, while the tentilla of all other known ctenophores elongate when relaxed. Euplokamis' tentilla have three types of movement that are used in capturing prey: they may flick out very quickly (in 40 to 60 milliseconds); they can wriggle, which may lure prey by behaving like small planktonic worms; and they coil round prey. The unique flicking is an uncoiling movement powered by contraction of the striated muscle. The wriggling motion is produced by smooth muscles, but of a highly specialized type. Coiling around prey is accomplished largely by the return of the tentilla to their inactive state, but the coils may be tightened by smooth muscle.[53]
There are eight rows of combs that run from near the mouth to the opposite end, and are spaced evenly round the body.[18] The "combs" beat in a metachronal rhythm rather like that of a Mexican wave.[54] From each balancer in the statocyst a ciliary groove runs out under the dome and then splits to connect with two adjacent comb rows, and in some species runs along the comb rows. This forms a mechanical system for transmitting the beat rhythm from the combs to the balancers, via water disturbances created by the cilia.[55]
Lobates
[edit]
The Lobata has a pair of lobes, which are muscular, cuplike extensions of the body that project beyond the mouth. Their inconspicuous tentacles originate from the corners of the mouth, running in convoluted grooves and spreading out over the inner surface of the lobes (rather than trailing far behind, as in the Cydippida). Between the lobes on either side of the mouth, many species of lobates have four auricles, gelatinous projections edged with cilia that produce water currents that help direct microscopic prey toward the mouth. This combination of structures enables lobates to feed continuously on suspended planktonic prey.[22]
Lobates have eight comb-rows, originating at the aboral pole and usually not extending beyond the body to the lobes; in species with (four) auricles, the cilia edging the auricles are extensions of cilia in four of the comb rows. Most lobates are quite passive when moving through the water, using the cilia on their comb rows for propulsion,[22] although Leucothea has long and active auricles whose movements also contribute to propulsion. Members of the lobate genera Bathocyroe and Ocyropsis can escape from danger by clapping their lobes, so that the jet of expelled water drives them back very quickly.[56] Unlike cydippids, the movements of lobates' combs are coordinated by nerves rather than by water disturbances created by the cilia, yet combs on the same row beat in the same Mexican wave style as the mechanically coordinated comb rows of cydippids and beroids.[55] This may have enabled lobates to grow larger than cydippids and to have less egg-like shapes.[54]
An unusual species first described in 2000, Lobatolampea tetragona, has been classified as a lobate, although the lobes are "primitive" and the body is medusa-like when floating and disk-like when resting on the sea-bed.[30]
Beroids
[edit]
The Beroida, also known as Nuda, have no feeding appendages, but their large pharynx, just inside the large mouth and filling most of the saclike body, bears "macrocilia" at the oral end. These fused bundles of several thousand large cilia are able to "bite" off pieces of prey that are too large to swallow whole – almost always other ctenophores.[57] In front of the field of macrocilia, on the mouth "lips" in some species of Beroe, is a pair of narrow strips of adhesive epithelial cells on the stomach wall that "zip" the mouth shut when the animal is not feeding, by forming intercellular connections with the opposite adhesive strip. This tight closure streamlines the front of the animal when it is pursuing prey.[58]
Other body forms
[edit]The Ganeshida has a pair of small oral lobes and a pair of tentacles. The body is circular rather than oval in cross-section, and the pharynx extends over the inner surfaces of the lobes.[22]
The Thalassocalycida, only discovered in 1978 and known from only one species,[59] are medusa-like, with bodies that are shortened in the oral-aboral direction, and short comb-rows on the surface furthest from the mouth, originating from near the aboral pole. They capture prey by movements of the bell and possibly by using two short tentacles.[22]
The Cestida ("belt animals") are ribbon-shaped planktonic animals, with the mouth and aboral organ aligned in the middle of opposite edges of the ribbon. There is a pair of comb-rows along each aboral edge, and tentilla emerging from a groove all along the oral edge, which stream back across most of the wing-like body surface. Cestids can swim by undulating their bodies as well as by the beating of their comb-rows. There are two known species, with worldwide distribution in warm, and warm-temperate waters: Cestum veneris ("Venus' girdle") is among the largest ctenophores – up to 1.5 meters (4.9 ft) long, and can undulate slowly or quite rapidly. Velamen parallelum, which is typically less than 20 centimeters (0.66 ft) long, can move much faster in what has been described as a "darting motion".[22][60]
Most Platyctenida have oval bodies that are flattened in the oral-aboral direction, with a pair of tentilla-bearing tentacles on the aboral surface. They cling to and creep on surfaces by everting the pharynx and using it as a muscular "foot". All but one of the known platyctenid species lack comb-rows.[22] Platyctenids are usually cryptically colored, live on rocks, algae, or the body surfaces of other invertebrates, and are often revealed by their long tentacles with many side branches, seen streaming off the back of the ctenophore into the current.
Reproduction and development
[edit]
Adults of most species can regenerate tissues that are damaged or removed,[61] although only platyctenids reproduce by cloning, splitting off from the edges of their flat bodies fragments that develop into new individuals.[22] Lab research on Mnemiopsis leidyi also show that when two individuals have parts of their bodies removed, they are able to fuse together, including their nervous and digestive systems, even when the two individuals are genetically different. A phenomenon that has so far only been found in comb jellies.[62]
The last common ancestor (LCA) of the ctenophores was hermaphroditic.[63] Some are simultaneous hermaphrodites, which can produce both eggs and sperm at the same time, while others are sequential hermaphrodites, in which the eggs and sperm mature at different times. There is no metamorphosis.[64] At least three species are known to have evolved separate sexes (dioecy); Ocyropsis crystallina and Ocyropsis maculata in the genus Ocyropsis and Bathocyroe fosteri in the genus Bathocyroe.[65] The gonads are located in the parts of the internal canal network under the comb rows, and eggs and sperm are released via pores in the epidermis. Fertilization is generally external, but platyctenids use internal fertilization and keep the eggs in brood chambers until they hatch. Self-fertilization has occasionally been seen in species of the genus Mnemiopsis,[22] and it is thought that most of the hermaphroditic species are self-fertile.[19]
Development of the fertilized eggs is direct; there is no distinctive larval form. Juveniles of all groups are generally planktonic, and most species resemble miniature adult cydippids, gradually developing their adult body forms as they grow. In the genus Beroe, however, the juveniles have large mouths and, like the adults, lack both tentacles and tentacle sheaths. In some groups, such as the flat, bottom-dwelling platyctenids, the juveniles behave more like true larvae. They live among the plankton and thus occupy a different ecological niche from their parents, only attaining the adult form by a more radical ontogeny[22] after dropping to the sea-floor.[19]
At least in some species, juvenile ctenophores appear capable of producing small quantities of eggs and sperm while they are well below adult size, and adults produce eggs and sperm for as long as they have sufficient food. If they run short of food, they first stop producing eggs and sperm, and then shrink in size. When the food supply improves, they grow back to normal size and then resume reproduction. These features make ctenophores capable of increasing their populations very quickly.[19] Members of the Lobata and Cydippida also have a reproduction form called dissogeny; two sexually mature stages, first as larva and later as juveniles and adults. During their time as larva they are capable of releasing gametes periodically. After their first reproductive period is over they will not produce more gametes again until later. A population of Mertensia ovum in the central Baltic Sea have become paedogenetic, and consist solely of sexually mature larvae less than 1.6 mm.[66][67]
In Mnemiopsis leidyi, nitric oxide (NO) signaling is present both in adult tissues and differentially expressed in later embryonic stages suggesting the involvement of NO in developmental mechanisms.[68] The mature form of the same species is also able to revert back to the cydippid stage when triggered by environmental stressors.[69]
Colors and bioluminescence
[edit]
Most ctenophores that live near the surface are mostly colorless and almost transparent. However some deeper-living species are strongly pigmented, for example the species known as "Tortugas red"[70] (see illustration here), which has not yet been formally described.[19] Platyctenids generally live attached to other sea-bottom organisms, and often have similar colors to these host organisms.[19] The gut of the deep-sea genus Bathocyroe is red, which hides the bioluminescence of copepods it has swallowed.[56]
The comb rows of most planktonic ctenophores produce a rainbow effect, which is not caused by bioluminescence but by the scattering of light as the combs move.[19][71] Most species are also bioluminescent, but the light is usually blue or green and can only be seen in darkness.[19] However some significant groups, including all known platyctenids and the cydippid genus Pleurobrachia, are incapable of bioluminescence.[72]
When some species, including Bathyctena chuni, Euplokamis stationis and Eurhamphaea vexilligera, are disturbed, they produce secretions (ink) that luminesce at much the same wavelengths as their bodies. Juveniles will luminesce more brightly in relation to their body size than adults, whose luminescence is diffused over their bodies. Detailed statistical investigation has not suggested the function of ctenophores' bioluminescence nor produced any correlation between its exact color and any aspect of the animals' environments, such as depth or whether they live in coastal or mid-ocean waters.[73]
In ctenophores, bioluminescence is caused by the activation of calcium-activated proteins named photoproteins in cells called photocytes, which are often confined to the meridional canals that underlie the eight comb rows. In the genome of Mnemiopsis leidyi ten genes encode photoproteins. These genes are co-expressed with opsin genes in the developing photocytes of Mnemiopsis leidyi, raising the possibility that light production and light detection may be working together in these animals.[74]
Ecology
[edit]
Distribution
[edit]Ctenophores are found in most marine environments: from polar waters at −2 °C to the tropics at 30 °C; near coasts and in mid-ocean; from the surface waters to the ocean depths at more than 7000 meters.[75] The best-understood are the genera Pleurobrachia, Beroe and Mnemiopsis, as these planktonic coastal forms are among the most likely to be collected near shore.[31][56] No ctenophores have been found in fresh water.
In 2013 Mnemiopsis was recorded in lake Birket Qarun, and in 2014 in lake El Rayan II, both near Faiyum in Egypt, where they were accidentally introduced by the transport of fish (mullet) fry. Though many species prefer brackish waters like estuaries and coastal lagoons in open connection with the sea, this was the first record from an inland environment. Both lakes are saline, with Birket Qarun being hypersaline, and shows that some ctenophores can establish themselves in saline limnic environments without connection to the ocean. In the long run it is not expected the populations will survive. The two limiting factors in saline lakes are availability of food and a varied diet, and high temperatures during hot summers. Because a parasitic isopod, Livoneca redmanii, was introduced at the same time, it is difficult to say how much of the ecological impact of invasive species is caused by the ctenophore alone.[76][77]
Ctenophores may be abundant during the summer months in some coastal locations, but in other places, they are uncommon and difficult to find.
In bays where they occur in very high numbers, predation by ctenophores may control the populations of small zooplanktonic organisms such as copepods, which might otherwise wipe out the phytoplankton (planktonic plants), which are a vital part of marine food chains.
Prey and predators
[edit]Almost all ctenophores are predators – there are no vegetarians and only one genus that is partly parasitic.[56] If food is plentiful, they can eat 10 times their own weight per day.[78] While Beroe preys mainly on other ctenophores, other surface-water species prey on zooplankton (planktonic animals) ranging in size from the microscopic, including mollusc and fish larvae, to small adult crustaceans such as copepods, amphipods, and even krill. Members of the genus Haeckelia prey on jellyfish and incorporate their prey's nematocysts (stinging cells) into their own tentacles instead of colloblasts.[19] Ctenophores have been compared to spiders in their wide range of techniques for capturing prey – some hang motionless in the water using their tentacles as "webs", some are ambush predators like Salticid jumping spiders, and some dangle a sticky droplet at the end of a fine thread, as bolas spiders do. This variety explains the wide range of body forms in a phylum with rather few species.[56] The two-tentacled "cydippid" Lampea feeds exclusively on salps, close relatives of sea-squirts that form large chain-like floating colonies, and juveniles of Lampea attach themselves like parasites to salps that are too large for them to swallow.[56] Members of the cydippid genus Pleurobrachia and the lobate Bolinopsis often reach high population densities at the same place and time because they specialize in different types of prey: Pleurobrachia's long tentacles mainly capture relatively strong swimmers such as adult copepods, while Bolinopsis generally feeds on smaller, weaker swimmers such as rotifers and mollusc and crustacean larvae.[79]
Ctenophores used to be regarded as "dead ends" in marine food chains because it was thought their low ratio of organic matter to salt and water made them a poor diet for other animals. It is also often difficult to identify the remains of ctenophores in the guts of possible predators, although the combs sometimes remain intact long enough to provide a clue. Detailed investigation of chum salmon, Oncorhynchus keta, showed that these fish digest ctenophores 20 times as fast as an equal weight of shrimps, and that ctenophores can provide a good diet if there are enough of them around. Beroids prey mainly on other ctenophores. Some jellyfish and turtles eat large quantities of ctenophores, and jellyfish may temporarily wipe out ctenophore populations. Since ctenophores and jellyfish often have large seasonal variations in population, most fish that prey on them are generalists and may have a greater effect on populations than the specialist jelly-eaters. This is underlined by an observation of herbivorous fishes deliberately feeding on gelatinous zooplankton during blooms in the Red Sea.[80] The larvae of some sea anemones are parasites on ctenophores, as are the larvae of some flatworms that parasitize fish when they reach adulthood.[81]
Ecological impacts
[edit]Most species are hermaphrodites, and juveniles of at least some species are capable of reproduction before reaching the adult size and shape. This combination of hermaphroditism and early reproduction enables small populations to grow at an explosive rate.

Ctenophores may balance marine ecosystems by preventing an over-abundance of copepods from eating all the phytoplankton (planktonic plants),[82] which are the dominant marine producers of organic matter from non-organic ingredients.[83]
On the other hand, in the late 1980s the Western Atlantic ctenophore Mnemiopsis leidyi was accidentally introduced into the Black Sea and Sea of Azov via the ballast tanks of ships, and has been blamed for causing sharp drops in fish catches by eating both fish larvae and small crustaceans that would otherwise feed the adult fish.[82] Mnemiopsis is well equipped to invade new territories (although this was not predicted until after it so successfully colonized the Black Sea), as it can breed very rapidly and tolerate a wide range of water temperatures and salinities.[84] The impact was increased by chronic overfishing, and by eutrophication that gave the entire ecosystem a short-term boost, causing the Mnemiopsis population to increase even faster than normal[85] – and above all by the absence of efficient predators on these introduced ctenophores.[84] Mnemiopsis populations in those areas were eventually brought under control by the accidental introduction of the Mnemiopsis-eating North American ctenophore Beroe ovata,[86] and by a cooling of the local climate from 1991 to 1993,[85] which significantly slowed the animal's metabolism.[84] However the abundance of plankton in the area seems unlikely to be restored to pre-Mnemiopsis levels.[87]
In the late 1990s Mnemiopsis appeared in the Caspian Sea. Beroe ovata arrived shortly after, and is expected to reduce but not eliminate the impact of Mnemiopsis there. Mnemiopsis also reached the eastern Mediterranean in the late 1990s and now appears to be thriving in the North Sea and Baltic Sea.[19]
Taxonomy
[edit]The number of known living ctenophore species is uncertain since many of those named and formally described have turned out to be identical to species known under other scientific names. Claudia Mills estimates that there about 100–150 valid species that are not duplicates, and that at least another 25, mostly deep-sea forms, have been recognized as distinct but not yet analyzed in enough detail to support a formal description and naming.[70]
Early classification
[edit]Early writers combined ctenophores with cnidarians into a single phylum called Coelenterata on account of morphological similarities between the two groups. Like cnidarians, the bodies of ctenophores consist of a mass of jelly, with one layer of cells on the outside and another lining the internal cavity. In ctenophores, however, these layers are two cells deep, while those in cnidarians are only a single cell deep. Ctenophores also resemble cnidarians in relying on water flow through the body cavity for both digestion and respiration, as well as in having a decentralized nerve net rather than a brain. Genomic studies have suggested that the neurons of Ctenophora, which differ in many ways from other animal neurons, evolved independently from those of the other animals,[88] and increasing awareness of the differences between the comb jellies and the other coelentarata has persuaded more recent authors to classify the two as separate phyla. The position of the ctenophores in the evolutionary family tree of animals has long been debated, and the majority view at present, based on molecular phylogenetics, is that cnidarians and bilaterians are more closely related to each other than either is to ctenophores.
Modern taxonomy
[edit]
The traditional classification divides ctenophores into two classes, those with tentacles (Tentaculata) and those without (Nuda). The Nuda contains only one order (Beroida) and family (Beroidae), and two genera, Beroe (several species) and Neis (one species).[70]
The Tentaculata are divided into the following eight orders:[70]
- Cydippida, egg-shaped animals with long tentacles[22]
- Lobata, with paired thick lobes[22]
- Platyctenida, flattened animals that live on or near the sea-bed; most lack combs as adults, and use their pharynges as suckers to attach themselves to surfaces[22]
- Ganeshida, with a pair of small lobes round the mouth, but an extended pharynx like that of platyctenids[22]
- Cambojiida
- Cryptolobiferida
- Thalassocalycida, with short tentacles and a jellyfish-like "umbrella"[22]
- Cestida, ribbon-shaped and the largest ctenophores[22]
There are fossil genera is considered stem group.
Evolutionary history
[edit]Despite their fragile, gelatinous bodies, fossils thought to represent ctenophores – apparently with no tentacles but many more comb-rows than modern forms – have been found in Lagerstätten as far back as the early Cambrian, about 515 million years ago. Nevertheless, a recent molecular phylogenetics analysis concludes that the common ancestor originated approximately 350 million years ago ± 88 million years ago, conflicting with previous estimates which suggests it occurred 66 million years ago after the Cretaceous–Paleogene extinction event.[13]
Fossil record
[edit]Because of their soft, gelatinous bodies, ctenophores are extremely rare as fossils, and fossils that have been interpreted as ctenophores have been found only in lagerstätten, places where the environment was exceptionally suited to the preservation of soft tissue. Until the mid-1990s only two specimens good enough for analysis were known, both members of the crown group, from the early Devonian (Emsian) period. Three additional putative species were then found in the Burgess Shale and other Canadian rocks of similar age, about 505 million years ago in the mid-Cambrian period. All three lacked tentacles but had between 24–80 comb rows, far more than the eight typical of living species. They also appear to have had internal organ-like structures unlike anything found in living ctenophores. One of the fossil species first reported in 1996 had a large mouth, apparently surrounded by a folded edge that may have been muscular.[3] Evidence from China a year later suggests that such ctenophores were widespread in the Cambrian, but perhaps very different from modern species – for example one fossil's comb-rows were mounted on prominent vanes.[89] The youngest fossil of a species outside the crown group is the species Daihuoides from the late Devonian, which belongs to a basal group that was assumed to have gone extinct more than 140 million years earlier.[90]
The Ediacaran Eoandromeda could putatively represent a comb jelly.[4] It has eightfold symmetry, with eight spiral arms resembling the comblike rows of a ctenophore. If it is indeed ctenophore, it places the group close to the origin of the Bilateria.[91] The early Cambrian sessile frond-like fossil Stromatoveris, from China's Chengjiang lagerstätte and dated to about 515 million years ago, is very similar to Vendobionta of the preceding Ediacaran period. De-Gan Shu, Simon Conway Morris, et al. found on its branches what they considered rows of cilia, used for filter feeding. They suggested that Stromatoveris was an evolutionary "aunt" of ctenophores, and that ctenophores originated from sessile animals whose descendants became swimmers and changed the cilia from a feeding mechanism to a propulsion system.[92] Other Cambrian fossils that support the idea of ctenophores having evolved from sessile forms are Dinomischus, Daihua, Xianguangia and Siphusauctum which also lived on the seafloor, had organic skeletons and cilia-covered tentacles surrounding their mouth, which have been found by cladistic analysis as members of the ctenophore stem-group[93][94]
520 million-year-old Cambrian fossils also from Chengjiang in China show a now wholly extinct class of ctenophore, named "Scleroctenophora", that had a complex internal skeleton with long spines.[95] The skeleton also supported eight soft-bodied flaps, which could have been used for swimming and possibly feeding. One form, Thaumactena, had a streamlined body resembling that of arrow worms and could have been an agile swimmer.[5]
Relationship to other animal groups
[edit]The phylogenetic relationship of ctenophores to the rest of Metazoa is very important to our understanding of the early evolution of animals and the origin of multicellularity. It has been the focus of debate for many years. Ctenophores have been purported to be the sister lineage to the Bilateria,[96][97] sister to the Cnidaria,[98][99][100][101] sister to Cnidaria, Placozoa, and Bilateria,[102][103][104] and sister to all other animals.[9][105]
Walter Garstang in his book Larval Forms and Other Zoological Verses (Mülleria and the Ctenophore) even expressed a theory that ctenophores were descended from a neotenic Mülleria larva of a polyclad.
A series of studies that looked at the presence and absence of members of gene families and signalling pathways (e.g., homeoboxes, nuclear receptors, the Wnt signaling pathway, and sodium channels) showed evidence congruent with the latter two scenarios, that ctenophores are either sister to Cnidaria, Placozoa, and Bilateria or sister to all other animal phyla. [106] [107] [108] [109] Several more recent studies comparing complete sequenced genomes of ctenophores with other sequenced animal genomes have also supported ctenophores as the sister lineage to all other animals.[110][28][111][112] This position would suggest that neural and muscle cell types either were lost in major animal lineages (e.g., Porifera and Placozoa) or evolved independently in the ctenophore lineage.[110]
Other researchers have argued that the placement of Ctenophora as sister to all other animals is a statistical anomaly caused by the high rate of evolution in ctenophore genomes, and that Porifera (sponges) is the earliest-diverging animal taxon instead.[104][113][114][115][116] They also have extremely high rates of mitochondrial evolution,[117] and the smallest known RNA/protein content of the mtDNA genome in animals.[118] As such, the Ctenophora appear to be a basal diploblast clade. In agreement with the latter point, the analysis of a very large sequence alignment at the metazoan taxonomic scale (1,719 proteins totalizing c. 400 000 amino acid positions) showed that ctenophores emerge as the second-earliest branching animal lineage, and sponges are sister-group to all other multicellular animals.[8] Also, research on mucin genes, which allow an animal to produce mucus, shows that sponges have never had them while all other animals, including comb jellies, appear to share genes with a common origin.[119] And it has been revealed that despite all their differences, ctenophoran neurons share the same foundation as cnidarian neurons after findings shows that peptide-expressing neurons are probably ancestral to chemical neurotransmitters.[120]
Yet another study strongly rejects the hypothesis that sponges are the sister group to all other extant animals and establishes the placement of Ctenophora as the sister group to all other animals, and disagreement with the last-mentioned paper is explained by methodological problems in analyses in that work.[13] Neither ctenophores nor sponges possess HIF pathways,[121] their genome express only a single type of voltage-gated calcium channel unlike other animals which have three types,[122] and they are the only known animal phyla that lack any true hox genes.[28] A few species from other phyla; the nemertean pilidium larva, the larva of the Phoronid species Phoronopsis harmeri and the acorn worm larva Schizocardium californicum, do not depend on hox genes in their larval development either, but need them during metamorphosis to reach their adult form.[123][124][125] Innexin genes, which code for proteins used for intercellular communication in animals, also appears to have evolved independently in ctenophores.[126]
Relationships within Ctenophora
[edit]
| ||||||||||||||||||||||||||||
Since all modern ctenophores except the beroids have cydippid-like larvae, it has widely been assumed that their last common ancestor also resembled cydippids, having an egg-shaped body and a pair of retractable tentacles. Richard Harbison's purely morphological analysis in 1985 concluded that the cydippids are not monophyletic, in other words do not contain all and only the descendants of a single common ancestor that was itself a cydippid. Instead he found that various cydippid families were more similar to members of other ctenophore orders than to other cydippids. He also suggested that the last common ancestor of modern ctenophores was either cydippid-like or beroid-like.[128] A molecular phylogeny analysis in 2001, using 26 species, including 4 recently discovered ones, confirmed that the cydippids are not monophyletic and concluded that the last common ancestor of modern ctenophores was cydippid-like. It also found that the genetic differences between these species were very small – so small that the relationships between the Lobata, Cestida and Thalassocalycida remained uncertain. This suggests that the last common ancestor of modern ctenophores was relatively recent, and perhaps survived the Cretaceous–Paleogene extinction event 65.5 million years ago while other lineages perished. When the analysis was broadened to include representatives of other phyla, it concluded that cnidarians are probably more closely related to bilaterians than either group is to ctenophores but that this diagnosis is uncertain.[127] A more recent 2017 study corroberates the paraphyly of Cydippida but also finds that Lobata is paraphyletic with respect to Cestida. Beyond this though, there is still a lot of missing gaps in the ctenophore tree despite their intensive study. Several families and orders do not have any species with complete genomes and as such their placement remains undetermined.
See also
[edit]References
[edit]- ^ Chen, Jun-Yuan; Schopf, J. William; Bottjer, David J.; Zhang, Chen-Yu; Kudryavtsev, Anatoliy B.; Tripathi, Abhishek B.; et al. (April 2007). "Raman spectra of a Lower Cambrian ctenophore embryo from southwestern Shaanxi, China". Proceedings of the National Academy of Sciences of the United States of America. 104 (15): 6289–6292. Bibcode:2007PNAS..104.6289C. doi:10.1073/pnas.0701246104. PMC 1847456. PMID 17404242.
- ^ Stanley, G.D.; Stürmer, W. (9 June 1983). "The first fossil ctenophore from the Lower Devonian of West Germany". Nature. 303 (5917): 518–520. Bibcode:1983Natur.303..518S. doi:10.1038/303518a0. S2CID 4259485.
- ^ a b Conway Morris, S.; Collins, D.H. (29 March 1996). "Middle Cambrian Ctenophores from the Stephen Formation, British Columbia, Canada". Philosophical Transactions of the Royal Society B: Biological Sciences. 351 (1337): 279–308. Bibcode:1996RSPTB.351..279C. doi:10.1098/rstb.1996.0024.
- ^ a b Tang, F.; Bengtson, S.; Wang, Y.; Wang, X.L.; Yin, C.Y. (20 September 2011). "Eoandromeda and the origin of Ctenophora". Evolution & Development. 13 (5): 408–414. doi:10.1111/j.1525-142X.2011.00499.x. PMID 23016902. S2CID 28369431.
- ^ a b Shu, Degan; Zhang, Zhifei; Zhang, Fang; Sun, Ge; Han, Jian; Xiao, Shuhai; Ou, Qiang (July 2015). "A vanished history of skeletonization in Cambrian comb jellies". Science Advances. 1 (6): e1500092. Bibcode:2015SciA....1E0092O. doi:10.1126/sciadv.1500092. PMC 4646772. PMID 26601209.
- ^ Fowler, George Herbert (1911). . In Chisholm, Hugh (ed.). Encyclopædia Britannica. Vol. 7 (11th ed.). Cambridge University Press. p. 593.
- ^ World Register of Marine Species (database). VLIZ. 18 September 2019. Retrieved 19 February 2024.
- ^ a b Simion, Paul; Philippe, Hervé; Baurain, Denis; Jager, Muriel; Richter, Daniel J.; diFranco, Arnaud; et al. (2017). "A large and consistent phylogenomic dataset supports sponges as the sister group to all other animals". Current Biology. 27 (7): 958–967. Bibcode:2017CBio...27..958S. doi:10.1016/j.cub.2017.02.031. PMID 28318975.
- ^ a b Dunn, Casey W.; Hejnol, Andreas; Matus, David Q.; Pang, Kevin; Browne, William E.; Smith, Stephen A.; et al. (2008). "Broad phylogenomic sampling improves resolution of the animal tree of life". Nature. 452 (7188): 745–749. Bibcode:2008Natur.452..745D. doi:10.1038/nature06614. PMID 18322464. S2CID 4397099.
- ^ Baxevanis, Andreas D.; Martindale, Mark Q.; Mullikin, James C.; Wolfsberg, Tyra G.; Dunn, Casey W.; Haddock, Steven H.D.; et al. (13 December 2013). "The genome of the Ctenophore Mnemiopsis leidyi and its implications for cell type evolution". Science. 342 (6164): 1242592. doi:10.1126/science.1242592. PMC 3920664. PMID 24337300.
- ^ Pisani, Davide; Pett, Walker; Dohrmann, Martin; Feuda, Roberto; Rota-Stabelli, Omar; Philippe, Hervé; et al. (2015). "Genomic data do not support comb jellies as the sister group to all other animals". Proceedings of the National Academy of Sciences of the U.S.A. 112 (50): 15402–15407. Bibcode:2015PNAS..11215402P. doi:10.1073/pnas.1518127112. PMC 4687580. PMID 26621703.
- ^ Berwald, Juli (November 2017). Spineless: The science of jellyfish and the art of growing a backbone. Ivanyi, Rachel (illus.) (1st hdbk ed.). New York, NY: Riverhead Books (Penguin Random House). ISBN 978-0735211261. LCCN 2017005838.
- ^ a b c d Whelan, Nathan V.; Kocot, Kevin M.; Moroz, Tatiana P.; Mukherjee, Krishanu; Williams, Peter; Paulay, Gustav; et al. (November 2017). "Ctenophore relationships and their placement as the sister group to all other animals". Nature Ecology & Evolution. 1 (11): 1737–1746. Bibcode:2017NatEE...1.1737W. doi:10.1038/s41559-017-0331-3. PMC 5664179. PMID 28993654.
- ^ Halanych, Kenneth M.; Whelan, Nathan V.; Kocot, Kevin M.; Kohn, Andrea B.; Moroz, Leonid L. (9 February 2016). "Miscues misplace sponges". Proceedings of the National Academy of Sciences of the U.S.A. 113 (8): E946–E947. Bibcode:2016PNAS..113E.946H. doi:10.1073/pnas.1525332113. ISSN 0027-8424. PMC 4776479. PMID 26862177.
- ^ Telford, Maximilian J.; Moroz, Leonid L.; Halanych, Kenneth M. (January 2016). "A sisterly dispute". Nature. 529 (7586): 286–287. doi:10.1038/529286a. ISSN 1476-4687. PMID 26791714. S2CID 4447056.
- ^ Schultz, Darrin T.; Haddock, Steven H.D.; Bredeson, Jessen V.; Green, Richard E.; Simakov, Oleg; Rokhsar, Daniel S. (17 May 2023). "Ancient gene linkages support ctenophores as sister to other animals". Nature. 618 (7963): 110–117. Bibcode:2023Natur.618..110S. doi:10.1038/s41586-023-05936-6. ISSN 1476-4687. PMC 10232365. PMID 37198475.
- ^ Ryan, Joseph F.; Schnitzler, Christine E.; Tamm, Sidney L. (December 2016). "Meeting report of Ctenopalooza: The first international meeting of ctenophorologists". EvoDevo. 7 (1): 19. doi:10.1186/s13227-016-0057-3. hdl:1912/8430. S2CID 931968.
- ^ a b c d e f g h i j k l
Hinde, R.T. (1998). "The Cnidaria and Ctenophor". In Anderson, D.T. (ed.). Invertebrate Zoology. Oxford University Press. pp. 28–57. ISBN 978-0-19-551368-4. - ^ a b c d e f g h i j k l m n
Mills, C.E. "Ctenophores – some notes from an expert". Seattle, WA: University of Washington. Retrieved 5 February 2009. - ^ a b Ruppert, E.E.; Fox, R.S. & Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 111–124. ISBN 978-0-03-025982-1.
- ^ a b Seipel, K.; Schmid, V. (June 2005). "Evolution of striated muscle: Jellyfish and the origin of triploblasty". Developmental Biology. 282 (1): 14–26. doi:10.1016/j.ydbio.2005.03.032. PMID 15936326.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj
Ruppert, E.E.; Fox, R.S. & Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 182–195. ISBN 978-0-03-025982-1. - ^ Moroz, Leonid L.; Norekian, Tigran P. (16 August 2018). "Atlas of Neuromuscular Organization in the Ctenophore, Pleurobrachia bachei (A. Agassiz, 1860)". bioRxiv 10.1101/385435.
- ^ Viitasalo, S.; Lehtiniemi, M. & Katajisto, T. (2008). "The invasive ctenophore Mnemiopsis leidyi overwinters in high abundances in the subarctic Baltic Sea". Journal of Plankton Research. 30 (12): 1431–1436. doi:10.1093/plankt/fbn088.
- ^ Trumble, W.; Brown, L. (2002). "Ctenophore". Shorter Oxford English Dictionary. Oxford University Press.
- ^
Ruppert, E.E.; Fox, R.S. & Barnes, R.D. (2004). Invertebrate Zoology (7 ed.). Brooks / Cole. pp. 76–97. ISBN 978-0-03-025982-1. - ^ Bergquist, P.R. (1998). "Porifera". In Anderson, D.T. (ed.). Invertebrate Zoology. Oxford University Press. pp. 10–27. ISBN 978-0-19-551368-4.
- ^ a b c Moroz, Leonid L.; Kocot, Kevin M.; Citarella, Mathew R.; Dosung, Sohn; Norekian, Tigran P.; Povolotskaya, Inna S.; et al. (June 2014). "The ctenophore genome and the evolutionary origins of neural systems". Nature. 510 (7503): 109–114. Bibcode:2014Natur.510..109M. doi:10.1038/nature13400. PMC 4337882. PMID 24847885.
- ^ Exposito, J-Y.; Cluzel, C.; Garrone, R. & Lethias, C. (2002). "Evolution of collagens". The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 268 (3): 302–316. doi:10.1002/ar.10162. PMID 12382326. S2CID 12376172.
- ^ a b Horita, T. (March 2000). "An undescribed lobate ctenophore, Lobatolampea tetragona gen. nov. & spec. nov., representing a new family, from Japan". Zoologische Mededelingen. 73 (30): 457–464. Retrieved 3 January 2009.
- ^ a b Haddock, Steven H. D. (November 2004). "A golden age of gelata: past and future research on planktonic ctenophores and cnidarians". Hydrobiologia. 530–531 (1–3): 549–556. doi:10.1007/s10750-004-2653-9. S2CID 17105070.
- ^ Martindale, M. Q.; Henry, J. Q. (October 1999). "Intracellular Fate Mapping in a Basal Metazoan, the Ctenophore Mnemiopsis leidyi, Reveals the Origins of Mesoderm and the Existence of Indeterminate Cell Lineages". Developmental Biology. 214 (2): 243–257. doi:10.1006/dbio.1999.9427. PMID 10525332.
- ^ Afzelius, BA (1961). "The fine structure of the cilia from ctenophore swimming-plates". The Journal of Biophysical and Biochemical Cytology. 9 (2): 383–94. doi:10.1083/jcb.9.2.383. PMC 2224992. PMID 13681575.
- ^ Kreps, T. A.; Purcell, J. E. & Heidelberg, K. B. (June 1997). "Escape of the ctenophore Mnemiopsis leidyi from the scyphomedusa predator Chrysaora quinquecirrha". Marine Biology. 128 (3): 441–446. Bibcode:1997MarBi.128..441K. doi:10.1007/s002270050110. S2CID 32975367.
- ^ Mills, Claudia E. (February 1984). "Density is Altered in Hydromedusae and Ctenophores in Response to Changes in Salinity". The Biological Bulletin. 166 (1): 206–215. doi:10.2307/1541442. JSTOR 1541442.
- ^ Alien-like comb jellies have a nervous system like nothing ever seen before
- ^ The jellyfish with a nervous system that is causing a shiver in the scientific community
- ^ a b Burkhardt, Pawel; Colgren, Jeffrey; Medhus, Astrid; Digel, Leonid; Naumann, Benjamin; Soto-Angel, Joan; et al. (20 April 2023). "Syncytial nerve net in a ctenophore adds insights on the evolution of nervous systems". Science. 380 (6642): 293–297. Bibcode:2023Sci...380..293B. doi:10.1126/science.ade5645. hdl:11250/3149299. PMID 37079688. S2CID 258239574. Retrieved 24 April 2023.
- ^ Parry, Luke A.; Lerosey-Aubril, Rudy; Weaver, James C.; Ortega-Hernández, Javier (2021). "Cambrian comb jellies from Utah illuminate the early evolution of nervous and sensory systems in ctenophores". iScience. 24 (9): 102943. Bibcode:2021iSci...24j2943P. doi:10.1016/j.isci.2021.102943. PMC 8426560. PMID 34522849.
- ^ Pennisi, Elizabeth (10 January 2019). "The gluey tentacles of comb jellies may have revealed when nerve cells first evolved". Science. doi:10.1126/science.aaw6288. S2CID 92852830.
- ^ Did the ctenophore nervous system evolve independently?
- ^ Marlow, Heather; Tosches, Maria Antonietta; Tomer, Raju; Steinmetz, Patrick R.; Lauri, Antonella; Larsson, Tomas; Arendt, Detlev (29 January 2014). "Larval body patterning and apical organs are conserved in animal evolution". BMC Biology. 12 (1): 7. doi:10.1186/1741-7007-12-7. PMC 3939940. PMID 24476105.
- ^ Nielsen, Claus (15 February 2015). "Larval nervous systems: true larval and precocious adult". Journal of Experimental Biology. 218 (4): 629–636. doi:10.1242/jeb.109603. PMID 25696826. S2CID 3151957.
- ^ Nielsen, Claus (July 2019). "Early animal evolution: a morphologist's view". Royal Society Open Science. 6 (7): 190638. Bibcode:2019RSOS....690638N. doi:10.1098/rsos.190638. PMC 6689584. PMID 31417759.
- ^ Fox, Douglas (1 August 2017). "Aliens in our midst". Aeon. Retrieved 1 August 2017.
- ^ Hewitt, Olivia H.; Degnan, Sandie M. (13 February 2023). "Antioxidant enzymes that target hydrogen peroxide are conserved across the animal kingdom, from sponges to mammals". Scientific Reports. 13 (1): 2510. Bibcode:2023NatSR..13.2510H. doi:10.1038/s41598-023-29304-6. PMC 9925728. PMID 36781921. S2CID 256811787.
- ^ Norekian, Tigran P.; Moroz, Leonid L. (15 August 2019). "Neural system and receptor diversity in the ctenophore Beroe abyssicola". Journal of Comparative Neurology. 527 (12): 1986–2008. doi:10.1002/cne.24633. PMID 30632608.
- ^ Simmons, David K.; Martindale, Mark Q. (2015). "Ctenophora". Structure and Evolution of Invertebrate Nervous Systems. Oxford University Press. pp. 48–55. doi:10.1093/acprof:oso/9780199682201.003.0006. ISBN 9780199682201.
- ^ Jákely, Gáspár; Paps, Jordi; Nielsen, Claus (2015). "The phylogenetic position of ctenophores and the origin(s) of nervous systems". EvoDevo. 6 (1): 1. doi:10.1186/2041-9139-6-1. PMC 4406211. PMID 25905000.
- ^ "Five types of colloblast in a cydippid ctenophore, Minictena luteola Carré and Carré: An ultrastructural study and cytological interpretation". Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 341 (1298): 437–448. 1993. doi:10.1098/rstb.1993.0126.
- ^ Characterizing functional biodiversity across the phylum Ctenophora using physiological measurements
- ^ Mills, C. E.; Miller, R. L. (February 1984). "Ingestion of a medusa (Aegina citrea) by the nematocyst-containing ctenophore (Haeckelia rubra, formerly Euchlora rubra): Phylogenetic implications". Marine Biology. 78 (2): 215–221. Bibcode:1984MarBi..78..215M. doi:10.1007/BF00394704. S2CID 17714037.
- ^ Mackie, G. O.; Mills, C. E.; Singla, C. L. (March 1988). "Structure and function of the prehensile tentilla of Euplokamis (Ctenophora, Cydippida)". Zoomorphology. 107 (6): 319–337. doi:10.1007/BF00312216. S2CID 317017.
- ^ a b Craig, C.L.; Okubo, A. (April 1990). "Physical constraints on the evolution of ctenophore size and shape". Evolutionary Ecology. 4 (2): 115–129. Bibcode:1990EvEco...4..115C. doi:10.1007/BF02270909. S2CID 24584197.
- ^ a b Tamm, Sidney L. (1973). "Mechanisms of ciliary co-ordination in Ctenophores". Journal of Experimental Biology. 59: 231–245. doi:10.1242/jeb.59.1.231.
- ^ a b c d e f Haddock, S.H.D. (December 2007). "Comparative feeding behavior of planktonic ctenophores". Integrative and Comparative Biology. 47 (6): 847–853. doi:10.1093/icb/icm088. PMID 21669763.
- ^ Tamm, S. L.; Tamm, S. (1985). "Visualization of changes in ciliary tip configuration caused by sliding displacement of microtubules in macrocilia of the ctenophore Beroe". Journal of Cell Science. 79: 161–179. doi:10.1242/jcs.79.1.161. PMID 3914479.
- ^ Tamm, Sidney L.; Tamm, Signhild (1991). "Reversible epithelial adhesion closes the mouth of Beroe, a carnivorous marine jelly". Biological Bulletin. 181 (3): 463–473. doi:10.2307/1542367. JSTOR 1542367. PMID 29304670.
- ^ Gibbons, Mark J.; Richardson, Anthony J.; V. Angel, Martin; Buecher, Emmanuelle; Esnal, Graciela; Fernandez Alamo, Maria A.; et al. (June 2005). "What determines the likelihood of species discovery in marine holozooplankton: is size, range or depth important?". Oikos. 109 (3): 567–576. Bibcode:2005Oikos.109..567G. doi:10.1111/j.0030-1299.2005.13754.x.
- ^ Wrobel, David; Mills, Claudia (2003) [1998]. Pacific Coast Pelagic Invertebrates: A Guide to the Common Gelatinous Animals. Sea Challengers and Monterey Bay Aquarium. pp. 108. ISBN 978-0-930118-23-5.
- ^ Martindale, M. Q. (December 1986). "The ontogeny and maintenance of adult symmetry properties in the ctenophore, Mnemiopsis mccradyi". Developmental Biology. 118 (2): 556–576. doi:10.1016/0012-1606(86)90026-6. PMID 2878844.
- ^ Rapid physiological integration of fused ctenophores
- ^ Sasson, Daniel A.; Ryan, Joseph F. (December 2017). "A reconstruction of sexual modes throughout animal evolution". BMC Evolutionary Biology. 17 (1): 242. Bibcode:2017BMCEE..17..242S. doi:10.1186/s12862-017-1071-3. PMC 5717846. PMID 29207942.
- ^ Edgar, Allison; Ponciano, José Miguel; Martindale, Mark Q. (2022). "Ctenophores are direct developers that reproduce continuously beginning very early after hatching". Proceedings of the National Academy of Sciences of the U.S.A. 119 (18): e2122052119. Bibcode:2022PNAS..11922052E. doi:10.1073/pnas.2122052119. PMC 9170174. PMID 35476523.
- ^ Harbison, G. R.; Miller, R. L. (February 1986). "Not all ctenophores are hermaphrodites. Studies on the systematics, distribution, sexuality and development of two species of Ocyropsis". Marine Biology. 90 (3): 413–424. Bibcode:1986MarBi..90..413H. doi:10.1007/bf00428565. S2CID 83954780.
- ^ Reitzel, A. M.; Pang, K.; Martindale, M. Q. (2016). "Developmental expression of 'germline'- and 'sex determination'-related genes in the ctenophore Mnemiopsis leidyi". EvoDevo. 7: 17. doi:10.1186/s13227-016-0051-9. PMC 4971632. PMID 27489613.
- ^ Jaspers, C.; Haraldsson, M.; Bolte, S.; Reusch, T. B.; Thygesen, U. H.; Kiørboe, T. (2012). "Ctenophore population recruits entirely through larval reproduction in the central Baltic Sea". Biology Letters. 8 (5): 809–12. doi:10.1098/rsbl.2012.0163. PMC 3440961. PMID 22535640.
- ^ Moroz, Leonid; Mukherjee, Krishanu; Romanova, Daria (2023). "Nitric oxide signaling in ctenophores". Front. Neurosci. 17: 1125433. doi:10.3389/fnins.2023.1125433. PMC 10073611. PMID 37034176.
- ^ This Benjamin Button-like Jellyfish Can Age in Reverse, From Adult to Juvenile
- ^ a b c d Mills, C. E. (May 2007). "Phylum Ctenophora: list of all valid scientific names". Retrieved 10 February 2009.
- ^ Welch, Victoria; Vigneron, Jean Pol; Lousse, Virginie; Parker, Andrew (14 April 2006). "Optical properties of the iridescent organ of the comb-jellyfish Beroë cucumis (Ctenophora)". Physical Review E. 73 (4): 041916. Bibcode:2006PhRvE..73d1916W. doi:10.1103/PhysRevE.73.041916. PMID 16711845.
- ^ Haddock, S.H.D.; Case, J.F. (December 1995). "Not all ctenophores are bioluminescent: Pleurobrachia". Biological Bulletin. 189 (3): 356–362. doi:10.2307/1542153. JSTOR 1542153. PMID 29244577.
- ^ Haddock, S.H.D.; Case, J.F. (8 April 1999). "Bioluminescence spectra of shallow and deep-sea gelatinous zooplankton: Ctenophores, medusae and siphonophores". Marine Biology. 133 (3): 571–582. Bibcode:1999MarBi.133..571H. doi:10.1007/s002270050497. S2CID 14523078.
- ^ Schnitzler, Christine E.; Pang, Kevin; Powers, Meghan L.; Reitzel, Adam M.; Ryan, Joseph F.; Simmons, David; et al. (2012). "Genomic organization, evolution, and expression of photoprotein and opsin genes in Mnemiopsis leidyi: a new view of ctenophore photocytes". BMC Biology. 10 (1): 107. doi:10.1186/1741-7007-10-107. PMC 3570280. PMID 23259493.
- ^ Winnikoff, Jacob R.; Haddock, Steven H. D.; Budin, Itay (1 November 2021). "Depth- and temperature-specific fatty acid adaptations in ctenophores from extreme habitats". Journal of Experimental Biology. 224 (21): jeb242800. doi:10.1242/jeb.242800. PMC 8627573. PMID 34676421.
- ^ el-Shabrawy, Gamal; Dumont, Henri (2016). "First record of a ctenophore in lakes: The comb-jelly Mnemiopsis leidyi (A. Agassiz, 1865) invades the Fayum, Egypt". BioInvasions Records. 5 (1): 21–24. doi:10.3391/bir.2016.5.1.04. S2CID 90377075.
- ^ Mohammed-Geba, Khaled; Sheir, Sherin; Aguilar, Robert; Ogburn, Matthew; Hines, Anson; Khalafallah, Hussain; et al. (2019). "Molecular and morphological confirmation of an invasive American isopod; Livoneca redmanii (Leach, 1818), from the Mediterranean region to Lake Qaroun, Egypt" (PDF). Egyptian Journal of Aquatic Biology & Fisheries. 23 (4): 251–273. doi:10.21608/ejabf.2019.54062.
- ^ Reeve, M.R.; Walter, M.A.; Ikeda, T. (July 1978). "Laboratory studies of ingestion and food utilization in lobate and tentaculate ctenophores 1: Ctenophore food utilization". Limnology and Oceanography. 23 (4): 740–751. doi:10.4319/lo.1978.23.4.0740.
- ^ Costello, J.H.; Coverdale, R. (October 1998). "Planktonic feeding and evolutionary significance of the lobate body plan within the Ctenophora". The Biological Bulletin. 195 (2): 247–248. doi:10.2307/1542863. JSTOR 1542863. PMID 28570175.
- ^ Bos, A.R.; Cruz-Rivera, E.; Sanad, A.M. (2016). "Herbivorous fishes Siganus rivulatus (Siganidae) and Zebrasoma desjardinii (Acanthuridae) feed on Ctenophora and Scyphozoa in the Red Sea". Marine Biodiversity. 47: 243–246. doi:10.1007/s12526-016-0454-9. S2CID 24694789.
- ^ Arai, Mary Needler (June 2005). "Predation on pelagic coelenterates: A review". Journal of the Marine Biological Association of the United Kingdom. 85 (3): 523–536. Bibcode:2005JMBUK..85..523A. doi:10.1017/S0025315405011458. S2CID 86663092.
- ^ a b Chandy, Shonali T.; Greene, Charles H. (July 1995). "Estimating the predatory impact of gelatinous zooplankton". Limnology and Oceanography. 40 (5): 947–955. Bibcode:1995LimOc..40..947C. doi:10.4319/lo.1995.40.5.0947.
- ^ Field, Christopher B.; Behrenfeld, Michael J.; Randerson, James T.; Falkowski, Paul (10 July 1998). "Primary production of the biosphere: Integrating terrestrial and oceanic components". Science. 281 (5374): 237–240. Bibcode:1998Sci...281..237F. doi:10.1126/science.281.5374.237. PMID 9657713.
- ^ a b c Purcell, Jennifer E.; Shiganova, Tamara A.; Decker, Mary Beth; Houde, Edward D. (2001). "The ctenophore Mnemiopsis in native and exotic habitats: U.S. estuaries versus the Black Sea basin". Hydrobiologia. 451 (1–3): 145–176. doi:10.1023/A:1011826618539. S2CID 23336715.
- ^ a b Oguz, T.; Fach, B. & Salihoglu, B. (December 2008). "Invasion dynamics of the alien ctenophore Mnemiopsis leidyi and its impact on anchovy collapse in the Black Sea". Journal of Plankton Research. 30 (12): 1385–1397. doi:10.1093/plankt/fbn094.
- ^ Bayha, K.M.; Harbison, R.; McDonald, J.H.; Gaffney, P.M. (2004). "Preliminary investigation on the molecular systematics of the invasive ctenophore Beroe ovata". In Dumont, H.; Shiganova, T.A.; Niermann, U. (eds.). Aquatic invasions in the Black, Caspian, and Mediterranean seas. NATO Science Series IV: Earth and Environmental Sciences. Vol. 35. Springer Netherlands. pp. 167–175. doi:10.1007/1-4020-2152-6_7. ISBN 978-1-4020-1866-4.
- ^ Kamburska, L. (2006). "Effects of Beroe c.f. ovata on gelatinous and other zooplankton along the Bulgarian Black Sea Coast". In Dumont, H.; Shiganova, T.A.; Niermann, U. (eds.). Aquatic Invasions in the Black, Caspian, and Mediterranean Seas. NATO Science Series IV: Earth and Environmental Sciences. Vol. 35. Springer Netherlands. pp. 137–154. doi:10.1007/1-4020-2152-6_5. ISBN 978-1-4020-1866-4.
- ^ "Comb jelly nseurons spark evolution debate". Quanta Magazine. 25 March 2015. Retrieved 12 June 2015.
- ^ Conway Morris, S. (2003). "The Cambrian "explosion" of metazoans and molecular biology: Would Darwin be satisfied?" (PDF). International Journal of Developmental Biology. 47 (7–8): 505–515. PMID 14756326. Archived from the original (PDF) on 24 December 2009. Retrieved 14 February 2009.
- ^ Klug, Christian; Kerr, Johanne; Lee, Michael S.Y.; Cloutier, Richard (2021). "A late-surviving stem-ctenophore from the Late Devonian of Miguasha (Canada)". Scientific Reports. 11 (1): 19039. Bibcode:2021NatSR..1119039K. doi:10.1038/s41598-021-98362-5. PMC 8463547. PMID 34561497.
- ^ Maxmen, Amy (7 September 2011). "Ancient sea jelly shakes evolutionary tree of animals". Scientific American. Retrieved 21 June 2018.
- ^ Shu, D.-G.; Conway Morris, S.; Han, J.; Li, Y.; Zhang, X.L.; Hua, H.; et al. (5 May 2006). "Lower Cambrian vendobionts from China and early diploblast evolution". Science. 312 (5774): 731–734. Bibcode:2006Sci...312..731S. doi:10.1126/science.1124565. PMID 16675697. S2CID 1235914.
- ^ Zhao, Yang; Vinther, Jakob; Parry, Luke A.; Wei, Fan; Green, Emily; Pisani, Davide; et al. (April 2019). "Cambrian sessile, suspension feeding stem-group Ctenophores and evolution of the comb jelly body plan". Current Biology. 29 (7): 1112–1125.e2. doi:10.1016/j.cub.2019.02.036. hdl:1983/40a6bcb8-a740-482c-a23c-7d563faea5c5. PMID 30905603.
- ^ Zhao, Yang; Hou, Xian-guang; Cong, Pei-yun (January 2023). "Tentacular nature of the 'column' of the Cambrian diploblastic Xianguangia sinica". Journal of Systematic Palaeontology. 21 (1). Bibcode:2023JSPal..2115787Z. doi:10.1080/14772019.2023.2215787. ISSN 1477-2019.
- ^ Mindy, Weisberger (10 July 2015). "Ancient jellies had spiny skeletons, no tentacles". livescience.com.
- ^ Conway Morris, Simon; Simonetta, Alberto M., eds. (1991). The Early Evolution of Metazoa and the Significance of Problematic Taxa. Cambridge University Press. p. 308. ISBN 978-0-521-11158-4.
- ^ Nielsen, Claus; Scharff, Nikolaj; Eibye-Jacobsen, Danny (April 1996). "Cladistic analyses of the animal kingdom". Biological Journal of the Linnean Society. 57 (4): 385–410. Bibcode:1996BJLS...57..385N. doi:10.1006/bijl.1996.0023.
- ^ Leuckart, Rudolf (1923). Ueber die Morphologie und die Verwandtschaftsverhältnisse der wirbellosen thiere. Ein Beitrag zur Charakteristik und Classification der thierischen Formen (in German). ISBN 978-1-245-56027-6.
- ^ Haeckel, Ernst (1896). Systematische Phylogenie der Wirbellosen Thiere. Invertebrata (in German). Vol. Part 2: Des Entwurfs Einer Systematischen Stammesgeschichte. ISBN 978-1-120-86850-3.
- ^ Hyman, Libbie Henrietta (1940). The Invertebrates. Vol. I Protozoa through Ctenophora. McGraw Hill. ISBN 978-0-07-031660-7.
- ^ Philippe, H.; Derelle, R.; Lopez, P.; Pick, K.; Borchiellini, C.; Boury-Esnault, N.; et al. (28 April 2009). "Phylogenomics Revives Traditional Views on Deep Animal Relationships". Current Biology. 19 (8): 706–712. Bibcode:2009CBio...19..706P. doi:10.1016/j.cub.2009.02.052. PMID 19345102. S2CID 15282843.
- ^ Wallberg, A.; Thollesson, M.; Farris, J.S.; Jondelius, U. (December 2004). "The phylogenetic position of the comb jellies (Ctenophora) and the importance of taxonomic sampling". Cladistics. 20 (6): 558–578. doi:10.1111/j.1096-0031.2004.00041.x. PMID 34892961. S2CID 86185156.
- ^ Collins, A.G. (2002). "Phylogeny of Medusozoa and the evolution of cnidarian life cycles". Journal of Evolutionary Biology. 15 (3): 418–432. doi:10.1046/j.1420-9101.2002.00403.x. S2CID 11108911.
- ^ a b Pick, K.S.; Philippe, H.; Schreiber, F.; Erpenbeck, D.; Jackson, D.J.; Wrede, P.; et al. (September 2010). "Improved phylogenomic taxon sampling noticeably affects non-bilaterian relationships". Molecular Biology and Evolution. 27 (9): 1983–1987. doi:10.1093/molbev/msq089. PMC 2922619. PMID 20378579.
- ^ Hejnol, A.; Obst, M.; Stamatakis, A.; Ott, M.; Rouse, G.W.; Edgecombe, G.D.; et al. (22 December 2009). "Assessing the root of bilaterian animals with scalable phylogenomic methods". Proceedings of the Royal Society B: Biological Sciences. 276 (1677): 4261–4270. doi:10.1098/rspb.2009.0896. PMC 2817096. PMID 19759036.
- ^ Ryan, J. F.; Pang, K.; Comparative Sequencing Program; Mullikin, J. C.; Martindale, M. Q.; Baxevanis, A. D.; NISC Comparative Sequencing Program (2010). "The homeodomain complement of the ctenophore Mnemiopsis leidyi suggests that Ctenophora and Porifera diverged prior to the ParaHoxozoa". EvoDevo. 1 (1): 9. doi:10.1186/2041-9139-1-9. PMC 2959044. PMID 20920347.
- ^ Reitzel, A. M.; Pang, K.; Ryan, J. F.; Mullikin, J. C.; Martindale, M. Q.; Baxevanis, A. D.; Tarrant, A. M. (2011). "Nuclear receptors from the ctenophore Mnemiopsis leidyi lack a zinc-finger DNA-binding domain: Lineage-specific loss or ancestral condition in the emergence of the nuclear receptor superfamily?". EvoDevo. 2 (1): 3. doi:10.1186/2041-9139-2-3. PMC 3038971. PMID 21291545.
- ^ Pang, K.; Ryan, J. F.; NISC Comparative Sequencing Program; Mullikin, J. C.; Baxevanis, A. D.; Martindale, M. Q. (2010). "Genomic insights into Wnt signaling in an early diverging metazoan, the ctenophore Mnemiopsis leidyi". EvoDevo. 1 (1): 10. doi:10.1186/2041-9139-1-10. PMC 2959043. PMID 20920349.
- ^ Liebeskind, B. J.; Hillis, D. M.; Zakon, H. H. (2011). "Evolution of sodium channels predates the origin of nervous systems in animals". Proceedings of the National Academy of Sciences of the U.S.A. 108 (22): 9154–9159. Bibcode:2011PNAS..108.9154L. doi:10.1073/pnas.1106363108. PMC 3107268. PMID 21576472.
- ^ a b Ryan, J.F.; Pang, K.; Schnitzler, C.E.; Nguyen, A.-D.; Moreland, R.T.; Simmons, D.K.; et al. (13 December 2013). "The Genome of the Ctenophore Mnemiopsis leidyi and its Implications for Cell Type Evolution". Science. 342 (6164): 1242592. doi:10.1126/science.1242592. PMC 3920664. PMID 24337300.
- ^ Whelan, Nathan V.; Kocot, Kevin M.; Moroz, Leonid L.; Halanych, Kenneth M. (5 May 2015). "Error, signal, and the placement of Ctenophora sister to all other animals". Proceedings of the National Academy of Sciences of the U.S.A. 112 (18): 5773–5778. Bibcode:2015PNAS..112.5773W. doi:10.1073/pnas.1503453112. PMC 4426464. PMID 25902535.
- ^ Borowiec, Marek L.; Lee, Ernest K.; Chiu, Joanna C.; Plachetzki, David C. (December 2015). "Extracting phylogenetic signal and accounting for bias in whole-genome data sets supports the Ctenophora as sister to remaining Metazoa". BMC Genomics. 16 (1): 987. doi:10.1186/s12864-015-2146-4. PMC 4657218. PMID 26596625.
- ^ Philippe, Hervé; Derelle, Romain; Lopez, Philippe; Pick, Kerstin; Borchiellini, Carole; Boury-Esnault, Nicole; et al. (April 2009). "Phylogenomics revives traditional views on deep animal relationships". Current Biology. 19 (8): 706–712. Bibcode:2009CBio...19..706P. doi:10.1016/j.cub.2009.02.052. PMID 19345102. S2CID 15282843.
- ^ Nosenko, Tetyana; Schreiber, Fabian; Adamska, Maja; Adamski, Marcin; Eitel, Michael; Hammel, Jörg; et al. (April 2013). "Deep metazoan phylogeny: When different genes tell different stories". Molecular Phylogenetics and Evolution. 67 (1): 223–233. Bibcode:2013MolPE..67..223N. doi:10.1016/j.ympev.2013.01.010. PMID 23353073.
- ^ Pisani, Davide; Pett, Walker; Dohrmann, Martin; Feuda, Roberto; Rota-Stabelli, Omar; Philippe, Hervé; et al. (15 December 2015). "Genomic data do not support comb jellies as the sister group to all other animals". Proceedings of the National Academy of Sciences of the U.S.A. 112 (50): 15402–15407. Bibcode:2015PNAS..11215402P. doi:10.1073/pnas.1518127112. PMC 4687580. PMID 26621703.
- ^ Kapli, Paschalia; Telford, Maximilian J. (11 December 2020). "Topology-dependent asymmetry in systematic errors affects phylogenetic placement of Ctenophora and Xenacoelomorpha". Science Advances. 6 (10): eabc5162. Bibcode:2020SciA....6.5162K. doi:10.1126/sciadv.abc5162. PMC 7732190. PMID 33310849.
- ^ Christianson, L.M.; Johnson, S.B.; Schultz, D.T.; Haddock, S.H. (2021). "Hidden diversity of Ctenophora revealed by new mitochondrial COI primers and sequences". Molecular Ecology Resources. 22 (1): 283–294. doi:10.1111/1755-0998.13459. PMC 9290464. PMID 34224654.
- ^ Kohn, A.B.; Citarella, M.R.; Kocot, K.M.; Bobkova, Y.V.; Halanych, K.M.; Moroz, L.L. (2011). "Rapid evolution of the compact and unusual mitochondrial genome in the ctenophore, Pleurobrachia bachei". Molecular Phylogenetics and Evolution. 63 (1): 203–207. doi:10.1016/j.ympev.2011.12.009. PMC 4024468. PMID 22201557.
- ^ Bakshani, Cassie R.; Morales-Garcia, Ana L.; Althaus, Mike; Wilcox, Matthew D.; Pearson, Jeffrey P.; Bythell, John C.; Burgess, J. Grant (4 July 2018). "Evolutionary conservation of the antimicrobial function of mucus: A first defence against infection". npj Biofilms and Microbiomes. 4 (1): 14. doi:10.1038/s41522-018-0057-2. PMC 6031612. PMID 30002868.
- ^
Okubo, Tomomi (8 August 2022). "Into the brain of comb jellies: Scientists explore the evolution of neurons". neurosciencenews.com (Press release). Okinawa, JA: Okinawa Institute of Science and Technology.
A study of combo jellies reveals the type of messenger that likely functioned in the ancestral nervous system.
— cites journal article: - ^ Mills, DB; Francis, WR; Vargas, S; Larsen, M; Elemans, CP; Canfield, DE; Wörheide, G (2018). "The last common ancestor of animals lacked the HIF pathway and respired in low-oxygen environments". eLife. 7. doi:10.7554/eLife.31176. PMC 5800844. PMID 29402379.
- ^ Gauberg, Julia; Abdallah, Salsabil; Elkhatib, Wassim; Harracksingh, Alicia N.; Piekut, Thomas; Stanley, Elise F.; Senatore, Adriano (25 December 2020). "Conserved biophysical features of the CaV2 presynaptic Ca2+ channel homologue from the early-diverging animal Trichoplax adhaerens". The Journal of Biological Chemistry. 295 (52): 18553–18578. doi:10.1074/jbc.RA120.015725. PMC 7939481. PMID 33097592.
- ^ Spradling, Allan (14 May 2012). "Relationships of early metazoans". Evolution and Development (PDF) (presentation slides). Evo-Devo research program. Washington, DC: Carnagie Science. p. 38. Archived from the original (PDF) on 2 March 2014. Retrieved 8 October 2024 – via carnegiescience.edu. — technical introduction and review of genomic research, evolution, and taxonomy
- ^ Hiebert, Laurel S.; Maslakova, Svetlana A. (2015). "Hox genes pattern the anterior-posterior axis of the juvenile but not the larva in a maximally indirect developing invertebrate, Micrura alaskensis (Nemertea)". BMC Biology. 13: 23. doi:10.1186/s12915-015-0133-5. PMC 4426647. PMID 25888821.
- ^ Gąsiorowski, Ludwik; Hejnol, Andreas (2019). "Hox gene expression during the development of the phoronid Phoronopsis harmeri". EvoDevo. 11: 2. bioRxiv 10.1101/799056. doi:10.1186/s13227-020-0148-z. PMC 7011278. PMID 32064072. S2CID 208578827.
- ^ Independent Innexin Radiation Shaped Signaling in Ctenophores
- ^ a b Podar, Mircea; Haddock, Steven H.D.; Sogin, Mitchell L.; Harbison, G. Richard (November 2001). "A molecular phylogenetic framework for the phylum Ctenophora using 18S rRNA genes". Molecular Phylogenetics and Evolution. 21 (2): 218–230. Bibcode:2001MolPE..21..218P. CiteSeerX 10.1.1.384.6705. doi:10.1006/mpev.2001.1036. PMID 11697917.
- ^ Harbison, G.R. (1985). "On the classification and evolution of the Ctenophora". In Conway Morris, S.; George, J.D.; Gibson, R.; Platt, H.M. (eds.). The Origins and Relationships of Lower Invertebrates. Clarendon Press. pp. 78–100. ISBN 978-0-19-857181-0.
Further reading
[edit]- Barnes, R.S.K.; Calow, P.; Olive, P.J.W.; Golding, D.W.; Spicer, J.I. (2001). The Invertebrates: A synthesis (3rd ed.). Blackwell. § 3.4.3, p. 63. ISBN 0-632-04761-5.
- Brusca, R.C.; Brusca, G.J. (2003). Invertebrates (2nd ed.). Sinauer Associates. ch. 9, p. 269. ISBN 0-87893-097-3.
- Moore, J. (2001). An Introduction to the Invertebrates. Cambridge University Press. § 5.4, p. 65. ISBN 0-521-77914-6.
- Schäfer, W. (1996). "Ctenophora, Rippenquallen". In Westheide, W.; Rieger, R. (eds.). Spezielle Zoologie. Vol. 1. Stuttgart, DE: Gustav Fischer Verlag.
- Wenzel, Bruno (1958). Glastiere des Meeres. Rippenquallen (Acnidaria). ISBN 3-7403-0189-9.
- Shasha, Mark (1992). Night of the Moonjellies. Simon & Schuster. ISBN 0-671-77565-0.
- Fox, Douglas (August 2017). "Aliens in our midst: What the ctenophore says about the evolution of intelligence". Aeon. London, UK / New York, NY / Melbourne, AU: Aeon Media Group – via Aeon.co.
The ctenophore's brain suggests that, if evolution began again, intelligence would re-emerge because nature repeats itself.
— in part a review of research up to Moroz et al. (2014)
External links
[edit]- Plankton chronicles (video). Archived from the original on 12 January 2012. — Short documentary films & photos
- "Jellyfish and comb jellies". Smithsonian Institution. 30 April 2018 – via ocean.si.edu. — overview at the Smithsonian Ocean portal
- "Ctenophores from the São Sebastião Channel, Brazil". Archived from the original on 4 February 2012 – via usp.br.
- Video of ctenophores at the National Zoo in Washington DC (video) – via YouTube.
- "Tree of animal life has branches rearranged by evolutionary biologists". sciencedaily.com (Press release). March 2008.
- Ctenophora. Guide to the marine zooplankton of south eastern Australia (fact sheet). Tasmanian Aquaculture & Fisheries Institute / Australian Antarctic Division. Australian Government Department of the Environment, Water, Heritage, and the Arts. June 2008. Archived from the original on 20 July 2008. Retrieved 8 October 2024 – via tafi.org.au.
{{cite report}}: CS1 maint: unfit URL (link) — includes pictures - The jelly connection. imagequest3d.com (mixed video & still images). — striking images, including a Beroe specimen attacking another ctenophore
- "In search of the first animals". National Geographic. 12 December 2013. Archived from the original on 12 December 2013.
