Soman

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
3,3-Dimethylbutan-2-yl methylphosphonofluoridate | |
| Other names
GD; Phosphonofluoridic acid, methyl-, 1, 2, 2-trimethylpropyl ester; 2-(Fluoromethylphosphoryl)oxy-3,3-dimethylbutane; Pinacolyl methylphosphonofluoridate; 1,2,2-Trimethylpropyl methylphosphonofluoridate; Methylpinacolyloxyfluorophosphine oxide; Pinacolyloxymethylphosphonyl fluoride; Pinacolyl methanefluorophosphonate; Methylfluoropinacolylphosphonate; Fluoromethylpinacolyloxyphosphine oxide; Methylpinacolyloxyphosphonyl fluoride; Pinacolyl methylfluorophosphonate; 1,2,2-Trimethylpropoxyfluoromethylphosphine oxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H16FO2P | |
| Molar mass | 182.175 g·mol−1 |
| Appearance | When pure, colorless liquid with odor resembling rotten fruit. With impurities, amber or dark brown, with odor of camphor oil. |
| Density | 1.022 g/cm3 |
| Melting point | −42 °C (−44 °F; 231 K) |
| Boiling point | 198 °C (388 °F; 471 K) |
| Moderate | |
| Vapor pressure | 0.40 mmHg (53 Pa) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Highly Toxic |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Soman (or GD, EA 1210, Zoman, PFMP, A-255, systematic name: O-pinacolyl methylphosphonofluoridate)[1] is an extremely toxic chemical substance. It is a nerve agent, interfering with normal functioning of the mammalian nervous system by inhibiting the enzyme cholinesterase. It is an inhibitor of both acetylcholinesterase and butyrylcholinesterase.[2] As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations according to UN Resolution 687. Its production is strictly controlled, and stockpiling is outlawed by the Chemical Weapons Convention of 1993 where it is classified as a Schedule 1 substance. Soman was the third of the so-called G-series nerve agents to be discovered along with GA (tabun), GB (sarin), and GF (cyclosarin).
When pure, soman is a volatile, corrosive, and colorless liquid with a faint odor like that of mothballs or rotten fruit.[3] More commonly, it is a yellow to brown color and has a strong odor described as similar to camphor. The LCt50 for soman is 70 mg·min/m3 in humans.
GD can be thickened for use as a chemical spray using an acryloid copolymer. It can also be deployed as a binary chemical weapon; its precursor chemicals are methylphosphonyl difluoride and a mixture of pinacolyl alcohol and an amine.[citation needed]
History
[edit]After World War I, during which mustard gas and phosgene were used as chemical warfare agents, the 1925 Geneva Protocol was signed in an attempt to ban chemical warfare. Nevertheless, research into chemical warfare agents and the use of them continued. In 1936 a new, more dangerous chemical agent was discovered when Gerhard Schrader of IG Farben in Germany isolated tabun (named GA for German Agent A by the United States), the first nerve agent, while developing new insecticides. This discovery was followed by the isolation of sarin (designated GB by the United States) in 1938, also discovered by Schrader.
During World War II, research into nerve agents continued in the United States and Germany. In summer 1944, soman, a colorless liquid with a camphor odor (designated GD by the United States), was developed by the Germans. Soman proved to be even more toxic than tabun and sarin. Nobel Laureate Richard Kuhn together with Konrad Henkel discovered soman during research into the pharmacology of tabun and sarin at the Kaiser Wilhelm Institute for Medical Research at Heidelberg.[4] This research was commissioned by the German Army. Soman was produced in small quantities at a pilot plant at the IG Farben factory in Ludwigshafen. It was never used in World War II.[5]
Producing or stockpiling soman was banned by the 1993 Chemical Weapons Convention. When the convention entered force, the parties declared worldwide stockpiles of 9,057 tonnes of soman. The stockpiles were destroyed by 2018.[6]
The crystal structure of soman complexed with acetylcholinesterase was determined by Millard et al. in 1999 by X-ray crystallography: 1som. Other solved acetylcholinesterase structures with soman bound to them include 2wfz, 2wg0 and 2wg1.
Structure and reactivity
[edit]
Soman (C(±)P(±)-soman) has four stereoisomers, each with a different toxicity, though largely similar. The stereoisomers are C(+)P(+)-soman, C(+)P(−)-soman C(−)P(−)-soman and C(−)P(+)-soman.[7][8]
Soman has a phosphonyl group with a fluoride and a (large) hydrocarbon covalently bound to it. The structure is thus similar to that of sarin, which has only a smaller hydrocarbon group attached (isopropyl). Because of the similarity between the chemical structures, the reactivity of the two compounds is almost the same. Soman and sarin will both react using the phospho oxygen group, which can bind to amino acids like serine.
Synthesis
[edit]The manufacture of soman is very similar to the manufacture of sarin. The difference is that the isopropanol from the sarin processes is replaced with pinacolyl alcohol:

Soman is synthesized by reacting pinacolyl alcohol with methylphosphonyl difluoride. The result of this reaction is the forming of soman which is described as “colorless liquid with a somewhat fruity odor.” The low vapor pressure of soman will also produce the volatile gas form of soman. Also, the acid hydrogen fluoride will form due to the elimination of fluoride and a proton. This acid is indirectly dangerous to humans. Skin contact with hydrogen fluoride will cause an immediate reaction with water which produces hydrofluoric acid.[5]
Mechanisms of action
[edit]Soman is an organophosphorus nerve agent with a mechanism of action similar to Tabun. Nerve agents inhibit acetylcholine esterase (AChE) by forming an adduct with the enzyme via a serine residue on that enzyme. These adducts may be decomposed hydrolytically or, for example, by the action of some oximes and thereby regenerate the enzyme. A second reaction type, one in which the enzyme–organophosphate (OP) complex undergoes a subsequent reaction, is usually described as "aging". Once the enzyme–OP complex has aged it is no longer regenerated by the common, oxime reactivators. The rate of this process is dependent on the OP. Soman is an OP that stimulates the rate of aging most rapidly decreasing the half-life to just a few minutes.
AChE is an enzyme involved with neurotransmission. Because of the severe decrease of the half-life of this enzyme, neurotransmission is abolished in a matter of minutes.[5]
Metabolism
[edit]Once taken up in the human body, soman not only inhibits AChE, but it is also a substrate for other esterases. Reaction of soman with these esterases allows for the detoxication of the compound. No metabolic toxification reactions are known for soman.
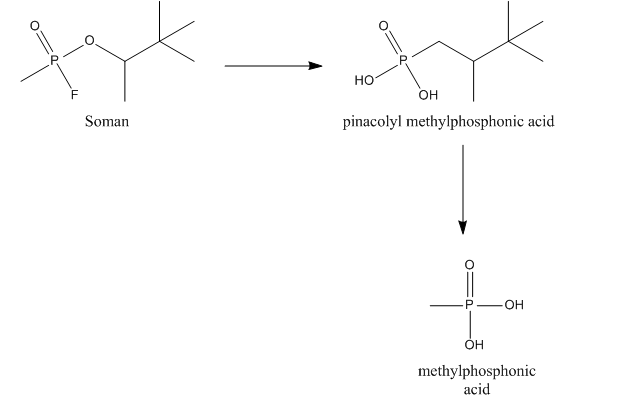
Soman can be hydrolyzed by a so-called A-esterase, more specific a diisopropylfluorophosphatase. This esterase, also called somanase, reacts with the anhydride bond between phosphorus and fluorine and accounts for the hydrolysis of the fluoride. Somanase also hydrolyses the methyl group of soman resulting in the formation of pinacolyl methylphosphonic acid (PMPA), which is a less potent AChE inhibitor.[9][10]
Soman can also bind to other esterases, e.g., AChE, cholinesterase (ChE) and carboxylesterases (CarbE). In this binding, soman loses its fluoride. After binding to AChE or ChE soman also loses its phosphoryl group, leading to the formation of methylphosphonic acid (MPA). Binding to CarbE reduce the total concentration of soman in the blood, thus resulting in a lower toxicity. Furthermore, CarbE are involved in the detoxication by hydrolysing soman to PMPA. So CarbE account for the detoxication of soman in two ways.[9][10]
The importance of the detoxication of soman after exposure was illustrated in experiments of Fonnum and Sterri (1981). They reported that only 5% of LD50 inhibited AChE in rats, resulting in acute toxic effects. This shows that metabolic reactions accounted for the detoxification of the remaining 95% of the dose.[11]
Signs and symptoms
[edit]As soman is closely related to compounds such as sarin, indications for a soman poisoning are relatively similar. One of the first observable signs of a soman poisoning is miosis. Some, but not all of the later indications are vomiting, extreme muscle pain and peripheral nervous system problems. Those symptoms show as soon as 10 minutes after exposure and may last for many days.[12]
In addition to the direct toxic effects on the nervous system, people exposed to soman may experience long-term effects, most of which are psychological. Subjects who were exposed to a small dose of soman suffered severe toxic effects; once treated, the subjects often developed depression, had antisocial thoughts, were withdrawn and subdued, slept restlessly and had bad dreams. These symptoms lasted six months after exposure but disappeared without lasting damage.[13]
Toxicity and efficacy
[edit]The LC50 of soman in air is estimated to be 70 mg min per m3. Compared with the LC50 value for a rat, the human lethal concentration is much lower (954.3 mg min/m3 versus 70 mg min/m3). For compounds such as soman, which may also be used as a weapon, often a fraction of the LC50 dose is where the first effects appear. Miosis is one of the first symptoms of soman intoxication and can be seen in doses of less than 1% of the LC50.[14]
Effects on animals
[edit]Experiments have been done in which rats were exposed to soman to test if behavioral effects could be seen at low doses without generating overt symptoms. Exposure of the rats to soman in a dose of less than 3 percent of the LD50 caused alterations of the behavior. The active avoidance of the exposed rats was less than the avoidance of non-exposed rats (two-way shuttlebox experiment). Also the motor coordination (hurdle-stepping task), open field behavior and active as well as passive avoidance behavior were affected. One can conclude that rats that are exposed to soman performed with less success in tasks that require motor activity as well as the function of higher structures of the central nervous system (CNS) on the same time. In this, soman has a predominantly central effect.
The knowledge of the effects of low doses of soman and other choline esterase inhibitors on rats could possibly be used to explain the relatively high incidence of airplane accidents due to errors of agricultural pilots. If this knowledge could be applied to humans, one could explain this high incidence with depressed choline esterase activity due to exposure to pesticides. It is not known whether the extrapolation from rats to humans can be made.[15]
References
[edit]- ^ [1] Archived 2013-09-12 at the Wayback Machine United States Senate, 103d Congress, 2d Session. (May 25, 1994). Material Safety Data Sheet -- Lethal Nerve Agents Somain (GD and Thickened GD). Retrieved Nov. 6, 2004.
- ^ Millard CB, Kryger G, Ordentlich A, et al. (June 1999). "Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level". Biochemistry. 38 (22): 7032–9. doi:10.1021/bi982678l. PMID 10353814. S2CID 11744952.
- ^ "CDC | Facts About Soman". emergency.cdc.gov. Centers for Disease Control and Prevention. Archived from the original on 2017-12-22. Retrieved 2018-03-20.
- ^ Schmaltz F (September 2006). "Neurosciences and research on chemical weapons of mass destruction in Nazi Germany". Journal of the History of the Neurosciences. 15 (3): 186–209. doi:10.1080/09647040600658229. ISSN 0964-704X. PMID 16887760. S2CID 46250604.
- ^ a b c Lukey, Brian J., Salem, Harry (2007). Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology, and Therapeutics. CRC Press. pp. 10–13. ISBN 9781420046618.
- ^ "Report of the OPCW on the Implementation of the Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction in 2017" (PDF). Organisation for the Prohibition of Chemical Weapons. 2018-11-19. p. 45. Retrieved 2024-02-09.
- ^ Langenberg JP, Spruit HE, Van Der Wiel HJ, Trap HC, Helmich RB, Bergers WW, Van Helden HP, Benschop HP (1998-07-01). "Inhalation Toxicokinetics of Soman Stereoisomers in the Atropinized Guinea Pig with Nose-Only Exposure to Soman Vapor". Toxicology and Applied Pharmacology. 151 (1): 79–87. doi:10.1006/taap.1998.8451. ISSN 0041-008X. PMID 9705889.
- ^ De Jong LP, Van Dijk C, Benschop HP (1988-08-01). "Hydrolysis of the four stereoisomers of soman catalyzed by liver homogenate and plasma from rat, guinea pig and marmoset, and by human plasma". Biochemical Pharmacology. 37 (15): 2939–2948. doi:10.1016/0006-2952(88)90279-1. ISSN 0006-2952. PMID 3395367.
- ^ a b Jokanović M (2001-09-25). "Biotransformation of organophosphorus compounds". Toxicology. 166 (3): 139–160. doi:10.1016/s0300-483x(01)00463-2. ISSN 0300-483X. PMID 11543910.
- ^ a b Jokanović M (2009-07-10). "Current understanding of the mechanisms involved in metabolic detoxification of warfare nerve agents". Toxicology Letters. 188 (1): 1–10. doi:10.1016/j.toxlet.2009.03.017. ISSN 1879-3169. PMID 19433263.
- ^ Fonnum, F., Sterri, S.H. (1981). "Factors modifying the toxicity of organophosphorus compounds including soman and sarin". Fundam. Appl. Toxicol. 1 (2): 143–147. doi:10.1016/S0272-0590(81)80050-4. PMID 7184780.
- ^ Sidell FR (1974). "Soman and Sarin: Clinical Manifestations and Treatment of Accident of Accidental Poisoning by Organophosphates". Clinical Toxicology. 7 (1): 1–17. doi:10.3109/15563657408987971. PMID 4838227.
- ^ Sidell FR (1974). "Soman and sarin: clinical manifestations and treatment of accidental poisoning by organophosphates". Clinical Toxicology. 7 (1): 1–17. doi:10.3109/15563657408987971. ISSN 0009-9309. PMID 4838227.
- ^ Bey TA, Sullivan JB, Walter FG (2001) Organophosphate and carbamate insecticides. In: Sullivan JB, Krieger GR (eds) Clinical environmental health and toxic exposures. Lippincott Williams & Williams, Philadelphia, pp 1046–1057
- ^ WOLTHUIS OL, VANWERSCH RA (1984-04-01). "Behavioral Changes in the Rat after Low Doses of Cholinesterase Inhibitors". Toxicological Sciences. 4 (2part2): 195–208. doi:10.1093/toxsci/4.2part2.195. ISSN 1096-6080. PMID 6724212.
External links
[edit]- United States Senate, 103d Congress, 2d Session (May 25, 1994). Material Safety Data Sheet -- Lethal Nerve Agents Somain (GD and Thickened GD) (Archived 2013-09-12 at the Wayback Machine). Retrieved Nov. 6, 2004.
- AChE inhibitors and substrates in Proteopedia
- 2wfz in Proteopedia
- 2wg0 in Proteopedia
- 2wg1 in Proteopedia
- 1som in Proteopedia
- https://somantoxicologia.wixsite.com/meusite (in Portuguese)

